Research Article
Basic Experiment on Thoracoscopic Aortic Cross-Clamping as an Alternative to Resuscitative Thoracotomy
Hisashi Matsumoto1,2*, Hiroshi Yasumatsu1,2, Kazuki Mashiko1,2, Takahiro Yagi1,2 and Hiroyuki
Yokota2
1Shock and Trauma Centre, Nippon Medical School Chiba Hokusoh Hospital, Japan
2Department of Emergency and Critical Care Medicine, Nippon Medical School, Japan
*Corresponding author: Hisashi Matsumoto, Department of Emergency and Critical Care Medicine , Nippon Medical School Chiba Hokusoh Hospital, 1715, Kamakari, Inzai, Chiba Prefecture, 270-1694, Japan
Published: 05 Nov, 2018
Cite this article as: Matsumoto H, Yasumatsu H,
Mashiko K, Yagi T, Yokota H. Basic
Experiment on Thoracoscopic Aortic
Cross-Clamping as an Alternative to
Resuscitative Thoracotomy. World J
Surg Surgical Res. 2018; 1: 1074.
Abstract
Background: Aortic Cross-Clamping (ACC) via Resuscitative Thoracotomy (RT) is performed
in patients with haemorrhagic shock not only to control bleeding, but also to redistribute the
limited blood volume to the coronary and cerebral arteries. In chest injury, RT, including clamshell
thoracotomy, is inevitable because haemostasis and repair of damaged organs are simultaneously
required. On the other hand, RT with AXC to control bleeding for intra-abdominal injury is
considered to be excessively invasive.
Objective: This basic experiment explored the possibility of a minimally invasive “thoracoscopic
AXC”.
Method: This experiment was conducted in accordance with the Declaration of Helsinki and the
animal experiment guidelines of the author’s institute. A Large Yorkshire pig (female) weighing
44.5 kg was placed under general anaesthesia (1% to 2% isoflurane) and a thoracoscope was inserted
through the left intercostal space. Approaching the posterior mediastinum along the left chest
wall, the descending aorta was identified and completely clamped with the inserted AXC forceps.
The difficulty of the procedure, total time until clamping, and problems encountered during the
procedure were recorded.
Results: The approach to the posterior mediastinum was relatively easy, but cross-clamping to the
intended position took some time due to obstruction by the pulmonary ligament. The total time
from insertion of the thoracoscope to completion of AXC was 3' 58" with no significant change in
the pig’s vital signs. The insertion ports of the thoracoscope and the AXC forceps had to be separate.
Conclusion: Although the acquisition of skills to directly and rapidly reach the descending aorta,
including setting the insertion points of the thoracoscope and the AXC forceps, is a significant
problem, this experiment revealed that this procedure is anatomically feasible. However, since
in humans the lung and the pulmonary ligament may hinder the approach to the posterior
mediastinum, repeated training may be necessary to shorten the maneuvering time. In addition,
development of a device integrating the thoracoscope and AXC forceps might be necessary.
Keywords: Aortic cross-clamping; Resuscitative thoracotomy; Haemorrhagic shock; Severe
trauma
Background
In lethal severe trauma, haemorrhage and brain injury are still the main causes of death. Patients
with brain injury frequently suffer from the difficult situation of returning to society even after lifesaving
treatment, but for those with exsanguinating haemorrhage, the possibility of survival and
return to work would be higher if rapid haemostasis and fluid resuscitation are achieved.
Aortic Cross-Clamping (ACC) has long been used as a measure for bleeding control in
devastating haemorrhage cases [1-4]. Cardiac arrest and secondary brain damage might be avoided
by saving time in completing haemostasis and enabling a limited blood volume to supply the
coronary and cerebral arteries [5,6]. Several reports have demonstrated the survival of patients who
underwent RT for abdominal injury, and in these cases, it was inferred that bleeding control by AXC
was successful [7-9].
In chest injury, RT, including clamshell thoracotomy, is inevitable because haemostasis and
repair of damaged organs are simultaneously required. On the other hand, RT combined with AXC to control bleeding in intra-abdominal injury is regarded as an
excessively invasive manoeuvre. Approach to the descending aorta
can be made quickly and reliably via RT, when there is no adhesion
in the thoracic cavity, however, performing RT in the case of extra
thoracic injuries is equivalent to adding an AIS (abbreviated injury
score) of 3 of chest trauma, which must be a significant invasion to
the patient. In fact, after the improvement of hemodynamics, bleeding
from the chest wound of RT sometimes persists, requiring repeated
thoracotomies and excessive use of blood transfusion. Therefore, to
maintain the effects of AXC and to overcome its weak points, a basic
experiment was conducted to explore the possibility of a minimally
invasive “thoracoscopic AXC” procedure.
Figure 1
Figure 2
Figure 2
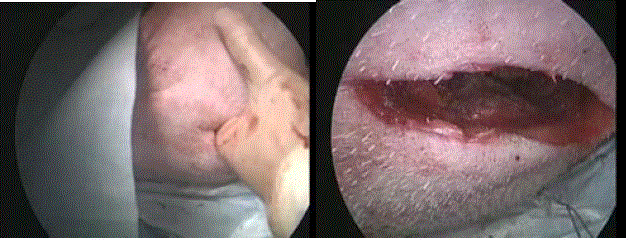
A small open thoracic incision was made just sufficiently large to
insert three fingers, in order to insert AXC forceps.
Figure 3
Methods and Materials
This experiment was conducted in accordance with the
Declaration of Helsinki and the animal experiment guidelines of the
author’s institute. A Large Yorkshire pig (female) weighing 44.5 kg
was intubated with administering 4.0 ml of pentobarbital after 24-hr
fasting and placed under general anaesthesia (1% to 2% isoflurane).
Anaesthesia was maintained with 2% isoflurane and muscle relaxant,
and a vital sign was monitored intraoperativelybya pulse oximeter
and electrocardiograph.
Two ports for inserting a thoracoscope and hand instrument for
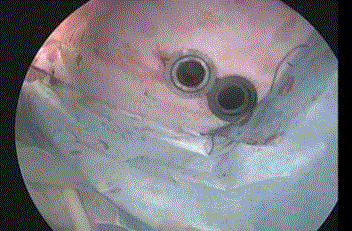
aortic clamping were placed on the left chest (Figure1). Separately
from those ports, a small open thoracic incision, just large enough
to insert three fingers, was made to insert AXC forceps (Figure 2).
A thoracoscope (IMAGE 1 HD Camera, KARL STORZ SE & Co.
KG, Tuttlingen) was inserted through one port. By approaching
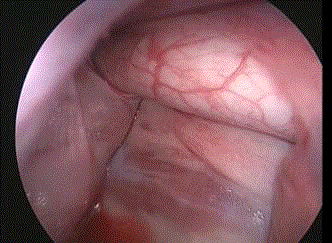
the posterior mediastinum while observing the diaphragm on the
left along the chest wall, the descending aorta was identified behind
the pulmonary ligament (Figure 3). Next, complete aortic clamping
was carried out with the inserted hand instrument (Endo Grasp TM,
COVIDIEN JAPAN Inc., Tokyo) via another port or with the AXC
forceps (Cooley’s aortic forceps, AESCULAPInc., USA) through the
open thoracic incision. The difficulty of the procedure, total time until
clamping, and problems encountered during the procedure were
recorded.
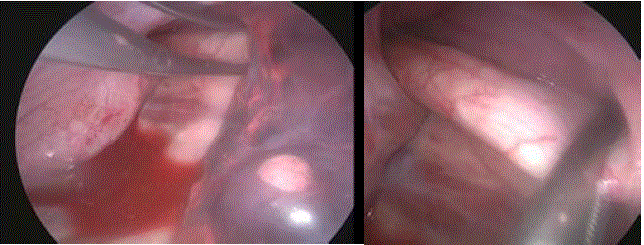
Figure 4
Figure 4
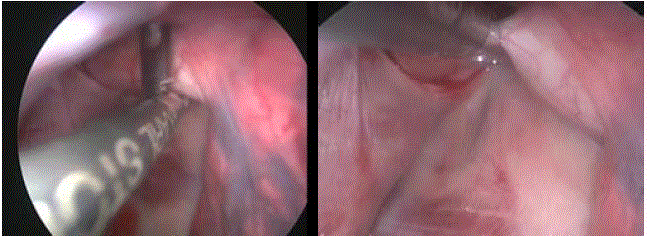
AXC by the hand instrument via the port. It was easier than using
AXC forceps, but since the hand device is small relative to the diameter of
the human aorta, there is a high possibility that the tip of the instrument could
slip from the aorta.
Figure 5
Figure 5
Using AXC forceps via the open thoracic wound. The time until
clamping using AXC forceps was prolonged because of obstruction by the
lung ligament, but strong clamping was achieved by large forceps.
Results
Through the thoracoscopic view, the field of operation in the
thoracic cavity was sufficiently secured, and visibility of the posterior
mediastinum was not problematic. The approach to the posterior
mediastinum was relatively easy, but cross-clamping to the intended
position took some time due to obstruction by the pulmonary
ligament.
It took roughly one minute to install two ports and conduct a
small thoracotomy, respectively. The same procedure was performed
twice, and the time from insertion of the thoracoscope to completion
of AXC by the hand instrument was 2' 50" the first time and 1' 25" the
second time (mean: 2' 08") (Figure 4). At this time, since the hand
device was sufficiently large relative to the diameter of the porcine
aorta, there was no possibility that the tip of the instrument could
slip from the aorta. The time needed to clamp using the AXC forceps
was 3' 38" the first time and 4' 14" the second time (mean: 3' 58"), and
strong clamping was achieved with large forceps (Figure 5).
There was no significant change in the pig’s vital signs during
the procedures. The insertion port of the thoracoscope and the AXC
forceps had to be separate.
Discussion
The implementation of AXC in trauma treatment was first
reported by Ledgerwood et al. [1]. AXC is an ultimate measure for
controlling haemorrhage and avoiding cardiac arrest in response to
subdiaphragmatic trauma, such as intra-abdominal injury or unstable
pelvic fracture, and is a dynamic procedure of considerable interest
to young doctors. However, patient conditions requiring AXC are
extremely serious, and AXC alone is notable to achievehaemostasis,
and the patient will not survive if bleeding after AXC does not
stop. Therefore, reports on the effect of AXC have not always been
favorable. This can also be inferred from many articles reporting
emergency department thoracotomy [10-12].
AXC sometimes achieves a dramatic result even if encompassing
several disadvantages. For example, spinal cord injury during
clamping and reperfusion injury after decamping are the most
common complications. The pathophysiologic condition for these
complications has been studied by many experiments [13-16], but
clinically preventive treatment has not yet been established. Since
these may coexist with AXC implementation, a “trade-off” between
the effect of AXC and complications must be considered when
performing AXC.
Apart from this issue, bleeding from the chest wall caused by
thoracotomy is another important complication of AXC. In RT under
unstable hemodynamics such as impending cardiac arrest, bleeding
from the opened chest wall is usually not regarded as a problem at that
time. However, when the circulation improves after the effect of AXC,
it may be difficult to stop bleeding, especially, from the dissected part
of the latissimus dorsi muscle deeply cut dorsally in order to widely
secure the thoracic cavity. This means that an invasion equivalent
to AIS of 3 of chest trauma is added by RT for AXC and should be
avoided especially with the concomitant complications of AXC.
Therefore, the possibility of thoracoscopic AXC was investigated
in order to implement “minimally invasive AXC” for extra thoracic
injury with impending cardiac arrest. Flaris et al. [17] investigated
RT enforcement time (skin to control of the heart wound) using
human cadavers [17], but no studies have examined the time to
AXC implementation. In this respect, we believe there is novelty
in this experiment. In this experiment, it was possible to carry out
thoracoscopic AXC in a relatively short time. However, based on our
experience, considering that a well-trained surgeon or emergency
physician can perform AXC via RT in around one minute, it can be
concluded that thoracoscopic AXC remains an inferior procedure.
Although quick cross-clamping was achieved in this experiment, this
could be attributed to the small diameter of the porcine descending
aorta which could be easily clamped with standard thoracoscopic
forceps. Accordingly, use of this device with the human aorta, which
has a large diameter, might not be appropriate. For this reason, a
dedicated larger device will be necessary for human use.
The manoeuvring time of thoracoscopic AXC must be shortened
further for this method to be practical. The time to reach the
descending aorta along the thoracic vertebrae initially was an
anatomical and technical issue, but it is possible that the time required
for this procedure is likely to decrease along with an increasing
degree of proficiency. Another concern was whether it is possible to
approach the descending aorta while successfully avoiding the left
lung. The porcine thorax is smaller than that of the human and it
was easy to approach in this experiment, but in humans the distance
from the chest wall to the posterior mediastinum is considerable,
so the expanding lung could become a barrier to approaching the
descending aorta. Clamping using Cooley’s aortic forceps was
unequivocal. However, the greatest challenge was obstruction by
the lung ligament to clamping the aorta, which prolonged the time
until the completion of clamping using AXC forceps. This anatomical
problem could be resolved by changing the direction in which the AXC
forceps are inserted. In addition, the fact that the thoracoscope and
the AXC forceps had to be inserted separately into the thoracic cavity
complicated the procedure and prolonged the time to complete AXC.
If a dedicated device can be developed to integrate the thoracoscope
and the guide way of the forceps, it would enable the insertions to
be conducted at the same position. In humans, conducting a smaller
thoracotomy by eliminating the need to make ports to insert both a
thoracoscope and forceps would shorten the manoeuvring time.
In recent years, the emergency medical system of dispatching
physicians to the scene and providing medical treatment has been
established. London’s Air Ambulance is actively implementing RT
in a prehospital setting and our facility also conducts prehospital
thoracotomy [18-22]. Thoracoscopic AXC may be a more effective
measure in the prehospital scene where bleeding for extrathoracic
haemorrhagemust be controlled with less equipment and a short
treatment time.
Limitations of this research were as follows. Firstly, because it
was a porcine experiment, it was difficult to evaluate the ease of the
anatomical approach to the thoracic descending aorta in humans.
Secondly, since it was a trial of an unusual procedure, it was not
possible to conduct a quantitative evaluation using many animals
from the viewpoint of animal protection.
Conclusion
In order to develop minimally invasive AXC against severe subdiaphragmatic injury involving fatal haemorrhage, the possibility of thoracoscopic AXC was investigated using animal experiments. However, in humans the lungs may hinder the approach to the posterior mediastinum, hence repeated training may be necessary to shorten the manoeuvring time. In the future, human trials should be conducted, including the development of specialized devices integrating the thoracoscope and AXC forceps.
References
- Ledgerwood AM, Kazmers M, Lucas CE. The role of thoracic aortic occlusion for massive hemoperitoneum. J Trauma. 1976;16(8):610-5.
- Brotman S, Oster-Granite M, Cox EF. Failure of cross clamping the thoracic aorta to control intra-abdominal bleeding. Ann Emerg Med. 1982;11(13):147-8.
- Millikan JS, Moore EE. Outcome of resuscitative thoracotomy and descending aortic occlusion performed in the operating room. J Trauma. 1984;24(5):387-92.
- Sheppard FR, Cothren CC, Moore EE, Orfanakis A, Ciesla DJ, Johnson JL, et al. Emergency department resuscitative thoracotomy for nontorso injuries. Surgery. 2006;139(4):574-6.
- Spence PA, Lust RM, Chitwood WR Jr, Iida H, Sun YS, Austin EH III. Transfemoral balloonaortic occlusion during open cardiopulmonary resuscitation improves myocardial and cerebral blood flow. J Surg Res. 1990;49(3):217-21.
- Wesley RC Jr, Morgan DB. Effect of continuous intra-aortic balloon inflation in canine open chest cardiopulmonary resuscitation. Crit Care Med. 1990;18(6):630-3.
- Lustenberger T, Labler L, Stover JF, Keel MJ. Resuscitative emergency thoracotomyin a Swiss trauma centre. Br J Surg. 2012;99(4):541-8.
- Moore EE, Knudson MM, Burlew CC, Inaba K, Dicker RA, Biffl WL, et al. Defining the limits of resuscitative emergency department thoracotomy: A contemporary Western Trauma Association perspective. J Trauma. 2011;70(2):334-9.
- Søreide K, Søikand H, Lossius HM, Vetrhus M, Søreide JA, Søreide E. Resuscitative emergency thoracotomy in a Scandinavian trauma hospital-Is it justified? Injury. 2007;38(1):34-42.
- Hunt PA, Greaves I, Owens WA. Emergency thoracotomy in thoracic trauma-a review. Injury. 2006;37(1):1-19.
- Pust GD, Namias N. Resuscitative thoracotomy. Int J Surg. 2016;33:202-8.
- Burlew CC, Moore EE, Moore FA, Coimbra R, McIntyre Jr RC, Davis W, et al. Western Trauma Association Critical Decisions in Trauma: Resuscitative thoracotomy. J Trauma Acute Care Surg. 2012;73(6):1359-63.
- Hill AC, Schecter WP, Mori H, Stevens MB, Husseni W, Lim RC, et al. The effect of verapamil on cerebral cortical and spinal cord blood flow during proximal descending thoracic aortic occlusion. J Trauma. 1988;28(8):1214-9.
- Marini CP, Levison J, Caliendo F, Nathan IM, Cohen JR. Control of proximal hypertension during aortic cross-clamping: its effect on cerebrospinal fluid dynamics and spinal cord perfusion pressure. Semin Thorac Cardiovasc Surg. 1998;10(1):51-6.
- Oyar EÖ, Kardes Ö, Korkmaz A, Ömeroĝlu S. Effects of vascular endothelial growth factor in ischemic spinal cord injury caused by aortic cross-clamping inrabbits. J Surg Res. 2009;151(1):94-9.
- Maier C, Scheuerle A, Hauser B, Schelzig H, Szabó C, Radermacher P, et al. The selective poly (ADP) ribose-polymerase 1 inhibitor INO1001reduces spinal cord injury during porcine aortic cross-clamping-induced ischemia/reperfusion injury. Intensive Care Med. 2007;33(5):845-50.
- Flaris AN, Simms ER, Prat N, Reynard F, Caillot JL, Voiglio EJ. Clamshell incision versus left anterolateral thoracotomy. Which one is faster when performing a resuscitative thoracotomy? The tortoise and the hare revisited. World J Surg. 2015;39(5):1306-11.
- Wise D, Davis G, Coats T, Lockey D, Hyde J, Good A. Emergency thoracotomy: “how to do it”. Emerg Med J. 2005;22(1):22-4.
- Deakin CD. From agonal to output: An ECG history of a successful pre-hospital thoracotomy. Resuscitation. 2007;75(3):525-9.
- Davis GE, Lockey DJ. Thirteen survivors of prehospital thoracotomy for penetrating trauma: A prehospital physician-performed resuscitation procedure that can yield good results. J Trauma. 2011;70(5):E75-8.
- Lockey DJ, Weaver AE, Davis GE. Practical translation of hemorrhage control techniques to the civilian trauma scene. Transfusion. 2013;53(1):17S-22S.
- Matsumoto H, Mashiko K, Hara Y, Kutsukata N, Sakamoto Y, Takei K, et al. Role of emergency field thoracotomy in the Japanese helicopter emergency medical service system. Resuscitation. 2009;80(11):1270-4.