Research Article
Intracorporeal Autologous Hepatocyte Matrix Implant for the Treatment of Chronic Liver Disease: A Modified Clinical Phase I Study
Hans U. Baer1*, Siufui Hendrawan2, Suryadi The3, Syafruddin AR. Lelosutan4, Salim G2, Toni Lindl5, Stephanie Mathes6, Ursula Graf-Hausner7, Ursula Weber1, Randall Watson8 and Barlian
Sutedja3
1Center of Abdominal Surgery, Hirslanden Clinic, University of Bern, Switzerland
2Tarumanagara Human Cell Technology Laboratory, Tarumanagara University, School of Medicine, Indonesia
3Department of Surgery, Gading Pluit Hospital, Indonesia
4Department of Internal Medicine, Gading Pluit Hospital, Indonesia
5IAZ - Institute for Applied Cell Culture, Germany
6University of Zurich, Center of Dental Medicine, Switzerland
7Zurich University of Applied Sciences, Tissue Engineering/Drug Development (TEDD), Switzerland
8Medical Writer, Limmatstrasse, Switzerland
*Corresponding author: Hans U. Baer, Center of Abdominal Surgery, Klinik Hirslanden, Witellikerstrasse 40, 8052 Zurich, University of Bern, Inselspital Bern, Switzerland
Published: 17 Oct, 2018
Cite this article as: Baer HU, Hendrawan S, The S,
Lelosutan SAR, Salim G, Lindl T, , et al.
Intracorporeal Autologous Hepatocyte
Matrix Implant for the Treatment of
Chronic Liver Disease: A Modified
Clinical Phase I Study. World J Surg
Surgical Res. 2018; 1: 1067.
Abstract
Background and Aim: Cultured human autologous hepatocyte matrix implants have been used to
treat chronic liver cirrhosis with some success and are a promising method to counter liver damage.
The options afforded by hepatocyte transplant are of special importance as this may prolong or
improve the lives of patients waiting for a transplant. We assessed the safety of implanted scaffold
cultured hepatocytes upon patient survival in a modified phase 1 trial.
Methods: We present here the first ethically approved in human study of autologous hepatocyte
matrix implantation for the treatment of predominantly viral hepatitis induced cirrhosis. The study
was performed in patients with longstanding liver disease at a single center in Jakarta, Indonesia. Liver
segments and pancreatic tissue from each patient were taken and processed to single hepatocytes
and islets of Langerhans, respectively. The single cells were co-seeded onto PLA scaffolds, cultured,
and transplanted back into the patient. We performed pre-implantation diagnosis and measured
clinical disease severity scores (CTP and MELD) and liver function (albumin, bilirubin, ammonia,
cholinesterase levels). In our small cohort, the procedure was generally safe.
Results: The small patient number prevented any statistical assessment of efficacy. For some
patients we observed clinically relevant improvements of liver function. Some patients showed
improvements in stamina, endurance to physical stress, reduced frequency of hospitalization and
incidence of encephalopathy, GI bleeding, ascites, and esophageal varices.
Conclusion: Although limited in power for statistical significance, the present work presents
sufficient data to warrant pursuing a phase 2 follow-up study.
Keywords: Chronic liver disease; Autologous hepatocyte matrix implant; PLLA matrix
Abbreviations
CTP: Child-Turcotte-Pugh; GI: Gastrointestinal; ICU: Intensive-Care Unit; MELD: Model for End-stage Liver Disease; PGA: Poly-Glycolic Acid; PLA: Poly-Lactic Acid
Introduction
Chronic liver cell injury resulting in liver cirrhosis has become a major health burden in many
societies. In western countries, alcohol abuse has been identified as a primary cause of such cirrhosis.
In Asian countries, viral hepatitis is a more prevalent factor leading to cirrhosis and eventual
hepatic failure. Patients suffering hepatic failure and cirrhosis-induced metabolic disorders are
known to present very complex cases [1]. Often, patients suffer from impaired coagulation, altered consciousness and cerebral function, a heightened risk of multiple
organ failure, and even sepsis.
In the western world liver transplantation remains the only
curative option in most cases [1]. Unfortunately, even in modern
and well-funded healthcare systems a severe shortage of donors
means that many patients die on waiting lists [2]. In many developing
nations, the sophisticated transplant programs that are common in
developed nations are in a fledgling state or do not exist. Consequently
the prognosis in terms of morbidity and mortality is poor depending
upon the regional situation [3]. A method to prolong life or improve
patient health would increase the likelihood of receiving a transplant
and could have resounding implications upon overall survival in this
patient group.
Hepatocyte transplantation is emerging as a promising method to
repair liver damage [4]. Early attempts used cells from heterologous
cell donors injected into the splenic or portal vein [5,6]. However,
poor cell engraftment and viability have been identified as limiting
factors in ‘traditional’ hepatocyte transplantation [1,7].
Attempts to improve engraftment and to implant cells that are
more durable and viable have resulted in advancements in tissue
engineering. In more recent years, such efforts have increased the
potential for implantation success of exogenous tissue matrices
seeded with autologous hepatocytes [8].
A number of these exogenous tissue matrices utilize Poly-Lactic
Acid (PLA) and Poly-Glycolic Acids (PLG) to form a temporary 3
dimensional scaffold to support cell growth and enable the generation
of in vitro tissue. PLA and PLG have a long history of clinical use as
components of sutures and implants. They are clinically tested and
safe, with minimal toxicity [9].
Typically, the three dimensional scaffolds are seeded with cells and
cultured to create an engineered tissue which can then be implanted.
Uyama et al. found that the viability of the hepatocyte tissue implants
can be greatly enhanced by co-seeding the hepatocytes with pancreatic
cells, more specifically islets of Langerhans, which showed the role of
hepatotropic stimulation in hepatocyte transplantation [10].
Animal studies
Uyama et al. used 3D PLA scaffolds to culture rat hepatocyte
cells [11]. This work showed superior cell density and function when
compared to 2D cultures. Moreover, engineered hepatocyte scaffolds,
when implanted into rats, were fully viable for a period of at least six
months [10].
It has been determined that for meaningful tissue function to be
achieved, the implants need to become properly vascularized, and
undertake metabolic exchange. The mesentery of the small bowel has
been found to provide a suitable implant location to establish such
vascularization and integration [12]. However, the number of animal
studies is limited and the generalizability of their findings unknown.
Therefore such studies are not necessarily transferable to human
applications [13].
Human implantation
Cultured human autologous hepatocyte matrix implants have
been used in non-viral cirrhotic patients [14]. In the only study
known to the authors, excluding the present work, 57 patients who
developed liver cirrhosis through alcoholism were treated with
autologous hepatocyte implants. For the majority of these patients,
liver-related blood values remained stable or improved 12 months
after implantation.
Undoubtedly, the results of this work look promising; however,
the study was limited to alcohol-related liver damage, little is known about the exact protocol, and it is unclear if the study was carried out
in a manner consistent with best practice.
Rationale
In the developing world currently, it is unlikely that cirrhotic
patients will receive a liver transplant. Being able to offer these
patients a “bridge to transplant”, that is, a method to prolong or
improve health and thereby increase the eventual likelihood of a
transplant would be especially welcome.
Here we present the first ethically approved in-human study
of autologous hepatocyte matrix implantation for the treatment of
predominantly viral hepatitis induced cirrhosis. This modified Phase
I study sets out to investigate safety, with the intention to extend to a
Phase II trial in the future.
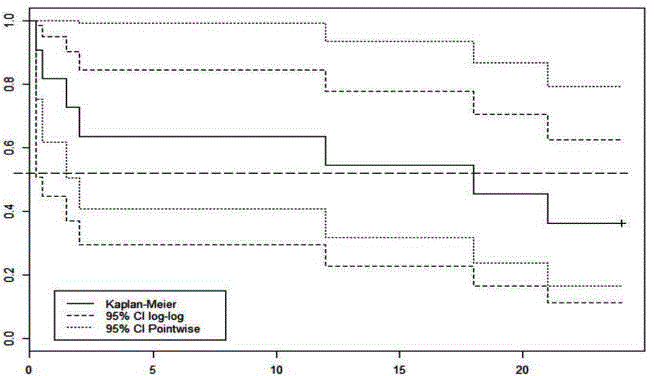
Figure 1
Figure 1
Kaplan-Meier plot showing overall survival and 95% confidence
intervals calculated using two different methods. The wide confidence
intervals prevent the determination of statistically significant conclusions on
survival.
Figure 2
Figure 3
Figure 4
Figure 5
Methods
Patients
Patients with longstanding liver disease under the care of two
specialists from a single center in Jakarta, Indonesia, were selected
using the inclusion criteria.
Informed consent & ethical approval
To ensure full patient understanding and allow patient family
consultation and considered decision making, patients were required
to provide informed consent on two separate occasions at least 4
weeks apart. Briefly, information brochures were given to potential
patients after the first consultation meeting. These materials included
the written description of the study, an integrated informed consent
agreement and the official declaration of approval from the Ethics
Committee.
Surgical technique and scaffold generation
The surgical procedure consisted of two separate procedures in
theatre under general anesthesia; firstly, the collection of liver and
pancreatic tissue, to provide hepatocytes and Islets of Langerhan’s
cells, and secondly, the implantation of the cultured implant matrices
into the small bowel mesentery.
Tissue harvest
Under strict sterile technique, a 4 cm × 4 cm × 4 cm liver
segment was removed from liver segment III. From the pancreas, a
biopsy of 3 mm × 3 mm × 3 mm was taken. Collected tissues were
treated according to the custom organ transplant protocol with
cold Custodiol® transplant solution and triple-bag sterile packing
with crushed ice, and transported to the laboratory in a monitored
cold box (0°C to 4°C). A transport time of less than two hours with
constant temperature is accepted as safe for tissue survival.
Engineered tissue scaffolds
The generation of the tissue scaffolds [15] will be the subject of a
secondary publication. Briefly, under strict Biosafety Level 2 conditions
at the Tarumanagara Human Cell Technology Laboratory, harvested
tissues were treated to liberate hepatocytes and islets of Langerhans
as described in the literature [14]. Cells, after quantification, were co
seeded onto 20 mm × 4 mm PLA disk scaffolds (Phrontier SARL, 2
rue Saint Clair, 76490 Caudebec en Caux, France) pre coated with
collagen type 1 to allow cell adherence. For each patient, 20 scaffolds
were seeded, each with 1-2 million cells in 300 μL William E medium
completed with 10% autologous patient serum. Seeded scaffolds were
cultured in 12-well cell culture plates with 0.7 mL William E with
10% autologous serum and incubated at 37°C with 5% CO2 and 95%
relative humidity for no less than 60 hr before implantation into the
patient.
Re-implantation
Approximately three days after the initial laparotomy, the
subcostal incision was reopened and the small bowel mesentery
moved into the wound. A 2 cm incision in the serosa was made and
a cavity with a well vascularized bed was formed to provide space for two implants. The serosa was closed with interrupted 5/0 sutures.
Fifteen to twenty implants were used per patient. The abdominal wall
was closed as soon as the matrices had been successfully inserted.
Assessment
Safety, the primary outcome, was directly assessed through
patient survival.
The following outcomes and pre-operative variables were
recorded for 24 months after the procedure or until death if earlier.
For some patients, detailed historical data were also available. The
following parameters were also recorded.
• Pre-implantation diagnosis
• Clinical disease severity scores
• CTP score
• MELD scores
• Albumin, bilirubin, ammonia and cholinesterase
Statistical analysis
The small number of patients precludes rigorous statistical
analyses relating to survival [11]. However, the primary objective of
this phase I trial was to determine safety and not efficacy.
Figure 6
Results
Fifty patients were screened in the Department of Surgery,
Gading Pluit Hospital, Indonesia, for possible inclusion in the study.
Eleven patients were included in the final cohort. Baseline assessment
data for each patient is given in Table 1.
Primary outcome-safety and survival
All patients survived the first operation (cell harvesting). Two
patients died following the second operation: one died one week
after surgery from severe Gastrointestinal (GI) bleeding caused
by esophageal varices, and the other 2 weeks post-surgery from
hepatorenal syndrome.
During the 24-month follow up, a further 5 patients died; none
were deemed procedure related. At 24 months, four patients remained
alive with good clinical indicators. Mortality and cause of death are
listed in Table 2. After 24 months, one of the surviving patients
returned liver function test results consistent with deteriorating
liver function caused by hepatitis C. Overall survival is shown in the
Kaplan-Meier plot in Figure 1 and 95% CIs were calculated using
both the point wise and the preferred log-log method.
Clinical and laboratory markers of liver function
Clinical assessments of patients were made throughout the follow
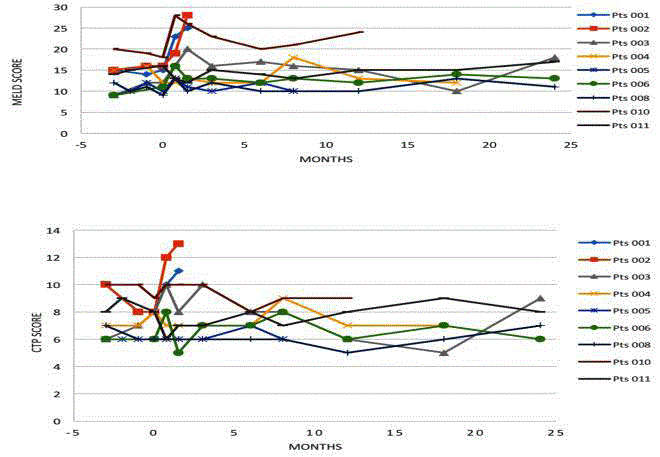
up period and detailed notes maintained. CTP and MELD scores for
each patient are shown in Figure 2. Individual CTP scores at 6, 12 and
24 months post-surgery were relatively stable compared to baseline.
Individual MELD scores at 6, 12 and 24 months were elevated in a
majority of patients.
The Karnofsky subjective scale showed a trend to slight
improvement in some of the patients (Figure 3).
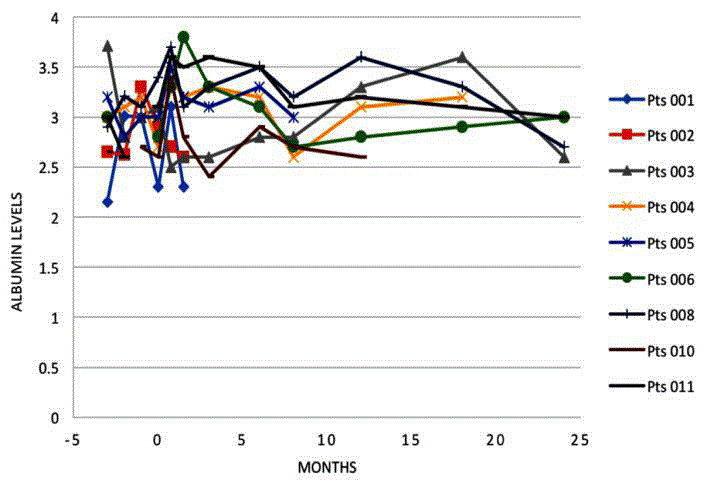
Albumin levels
Albumin levels are shown in Figure 4. Of the seven patients alive
at 6 months, six showed improved albumin levels compared with
time of admission. At 12 months, all living patients had improved
albumin levels [6], and at 24 months, one patient had improved
albumin levels, one was stable, and two had deteriorated.
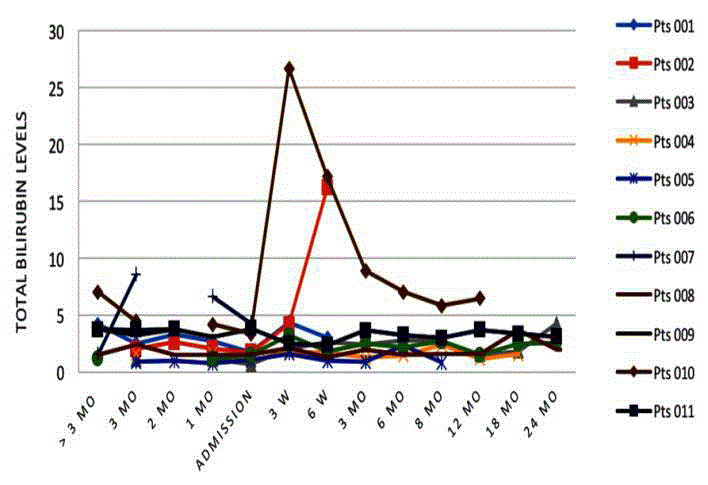
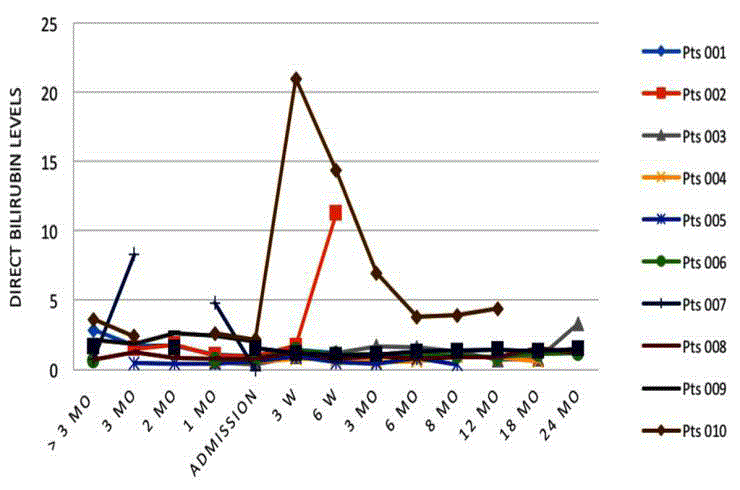
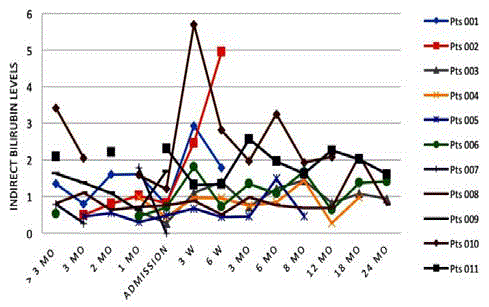
Bilirubin-total, direct and indirect (conjugated)
At six months, 5 of 7 patients had elevated total bilirubin levels
(Figure 5). Although there was some stabilization at 12 months, at
24 months, bilirubin levels were still above baseline in 3 of 4 patients.
Similarly, indirect and direct bilirubin levels fluctuated but remained
elevated in the majority of patients at all stages, with 3 of 4 patients
having elevated levels by 24 months (Figures 6 and 7).
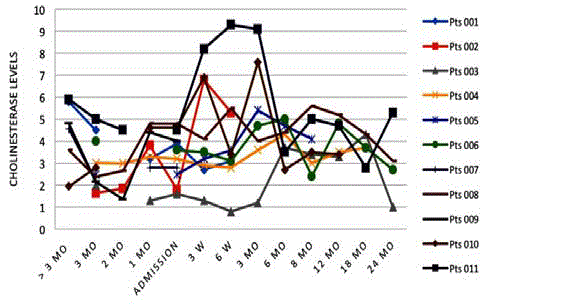
Cholinesterase
The fluctuation through time of cholinesterase levels is shown in
Figure 8 for each patient. In general, patients surviving at 18 months
showed general improvement in cholinesterase.
Ammonia
Levels fluctuated in the follow-up period, but were not
significantly elevated or lowered.
Other results
At 24 months, all patients still on study stated they had greater
stamina and better endurance to physical stress, although this was
not measured objectively. Subjective patient-reported observation
indicated a greater sense of well being, even amongst those who
eventually died during the study. Incidence of encephalopathy and GI
bleeding were also diminished among the remaining patients. And in
two patients there was improvement in ascites or esophageal varices.
Figure 7
Figure 8
Discussion
The liver is the central organ in metabolism. Impairment of liver function can have severe effects that, among other symptoms, may manifest as jaundice, esophageal varices and bleeding, portal hypertension, ascites, primary liver failure, cachexia and encephalopathy. The variety of clinical symptoms is linked to the central role of hepatocytes, which perform a multitude of tasks such as albumin and cholinesterase synthesis, conversion and storage of glucose, detoxification of drugs, and excretion of bile and bile soluble substances like bilirubin. In the course of research to find new techniques that might alleviate the severe shortage of donor livers, we tested here evaluation-blinded, non-randomized autologous hepatocyte matrix implantation as a method to redress the balance in treating liver disease without the need for liver transplantation.
Table 1
Table 1
Patient baseline assessment including histological results of the 11 patients, using the Metavir Scoring System
Table 2
Safety
Primary outcome
The primary outcome of this phase I trial was to assess the
safety of the autologous cultured hepatocyte matrix transplantation
technique in patients suffering from severe liver cirrhosis due
primarily to hepatitis. To fulfill this criterion, the implants must be
well tolerated and should not induce an inflammatory reaction or
malignant transformation of the implanted cells. Additionally, the
overall risk for patients should be analogous to, or lower than, that
of orthotopic liver transplantation, which is the alternative treatment.
The inclusion of an orthotropic liver transplant control group in this
setting was unfeasible due to the extremely limited availability of such
treatment in the study clinic. Further, the inclusion of placebo control
is ethically unsupportable in this setting.
Despite strict inclusion and exclusion criteria, seven patients
died due to hepatorenal syndrome, GI bleeding, and or liver failure.
Of these, five were disease related and two were considered to be
procedure-related by ethical committee review. Of the two procedurerelated
deaths, one was due to acute hepatorenal syndrome deemed
to have been triggered by hepatic insult induced by the surgery.
Surgical interventions in patients with higher MELD scores (≥ 15)
are very risky with estimates of 90-day mortality exceeding 25.4% for
all surgery types and even higher for procedures involving hepatic
resection [16]. The patient in question suffered from non-viral
hepatitis (the only case in this trial) and had a pre-operative MELD
score of 21, the highest in the cohort. All patients who died within 90
days of the procedure had a MELD score of 15 or greater.
The other procedure-related death occurred through fundus varices bleeding 1 week after the surgical procedure. The classification
of this patient death as procedure-related has been questioned, and
it might have been preventable under slightly different follow-up
protocol requiring closer GI monitoring.
Indeed, GI bleeding is of significant concern for many cirrhotic
patients as a result of portal hypertension. In our cohort, 8 of 11
patients had a history of upper GI bleeding, and one other patient
on the trial died from upper GI bleeding on day 42 post-implant.
This death was classified as disease related and not as a result of the
procedure. The results of the first seven patients were reviewed by
both the ethical committee and advisory board. It was decided to
continue with the study but to revise the exclusion criteria to exclude
patients with a MELD score above 15.
Overall, mortality was not directly correlated with the procedure
but the impact and burden of the procedure did influence the outcome
of some patients. We found no mortality for those patients in CTP A
but for CTP B, mortality was closely correlated with the MELD score.
Mortality was mostly seen in those with MELD score of 15 or above.
Finally, it is important to note the general level of illness amongst the
cohort. Of 50 patients screened for inclusion in the study, 13 were
initially selected, however two of these were subsequently excluded
after a secondary assessment and one of these patients died only one
day after being discharged following the second assessment.
Impact of viral load on mortality
Ten of the 11 patients in the study cohort suffered from viral
hepatitis: 3 type B and 7 type C. The inclusion criteria excluded
patients with cirrhosis and active chronic viral hepatitis. In normal
daily practice, many physicians set a viral load limit up to 103 to
104 iU/mL. Our cohort included patients with up to 3 × 105 iU/mL,
but we also included three hepatitis patients with undetectable or
extremely low viral counts. We saw no correlation between 30-day
mortality and viral load for either hepatitis B or hepatitis C. However,
we did observe indicators of constant inflammation through slightly
elevated SGOT/SGPT/Gamma GT levels, and we suspect viral load
to be a cause of this inflammation and to ultimately play a role in
continued liver fibrosis. In the case of hepatitis B, we were able to rely
on oral antivirus medication; however we were not so fortunate for
hepatitis C cases.
Post-mortem
Religious protocol prevented any post-mortem examinations.
Postmortem examinations may have revealed interesting data on
potential malignancy issues and on the ultimate long-term viability
of the implanted tissue.
Clinical Benefit
Overall survival
The performance of the procedure in terms of clinical benefit is
difficult to assess. This is primarily a result of the limited numbers
enrolled in the study. Overall survival, as distinct from procedurerelated
mortality, and shown in the Kaplan-Meier plot, suggests a
median survival of around 18 months, however the broad confidence
intervals associated with such a small cohort negate objective value of
any such figure.
Clinical indicators
Clinical indicators of general health and prognosis were stable and
did not, in general, show marked improvement. The CTP and MELD
scores were relatively stable; however a slight increase in MELD
scores was noticed across the cohort. It should be noted that the
baseline MELD scores for patients were relatively high with a median
of 15 (9-21) and as such, most patients would be expected to exhibit a
rise in MELD score over time due to natural disease progression. We
therefore believe the slow rise in MELD score is attributable to the
natural disease course and do not note any correlation of individual
patient’s MELD scores with their health state. It must be remembered
that the MELD score is not a measure of vitality, rather a measure of
the need for transplantation.
During the initial 3-month pre-operative period the CTP scores
decreased for 5 of 11 patients, and increased for two patients. This
decrease probably reflects overall improvement in liver function in
these patients and is likely due to the cyclic nature of the cirrhosis.
Patients were treated during the study with best practice clinical
support, standard medical therapy for hepatitis, and nutritive
control. Patients were monitored during the pre-treatment phase to
exclude those who were spontaneously recovering. The CTP score is
a clinical score however and not a prognostic score, and as such all
patients enrolled had high MELD scores and were in relatively poor
condition. Despite the slight improvement in the CTP score for some
patients, the improvement was not of sufficient magnitude to warrant
exclusion from the study.
Laboratory values
Bilirubin levels are an indicator for bile excretion and therefore
liver function. All patients showed a temporary increase in bilirubin
immediately following surgery, a common occurrence following
any surgical intervention in cirrhotic patients. Aside from 2 patients
who showed sudden increases in total bilirubin after surgery, one
subsequently died at 6 weeks and the other recovered. Bilirubin levels
remained mostly stable throughout the course of the study with the
highest increase being a doubling of the baseline level. When examined
in further detail, direct bilirubin, reflecting the true excretion function
of bile from the liver, remained at or around baseline levels, except in
the two aforementioned patients. One further patient showed a late
increase in direct bilirubin from 18-24 months coupled with a general
deterioration of health. Indirect bilirubin levels fluctuated slightly
more than direct bilirubin. Except for the two cases of significant
elevation, bilirubin fluctuations were not clinically relevant.
Serum albumin levels gradually improved across all patients
who survived beyond 6 months; however a general trend for stable
or slightly decreasing serum albumin developed after 12-18 months
amongst surviving patients. One patient, with a 20 year history of
hepatitis B requiring repeated albumin infusions prior to treatment,
showed continuously improving albumin levels after treatment
up to the end of the study where a sharp decrease in albumin and
concomitant increase in bilirubin and rising hepatitis C viral
levels were recorded. Overall, the increase in albumin is clinically
significant in the first year; however, the limited cohort does not allow
statistically significant conclusions to be made.
Laboratory results for ammonia, as a reflection of liver
metabolism and excretory function, fluctuated throughout the course
of the study but remained within allowable levels and appeared to
show no correlation with patient daily performance. Cholinesterase
as an indicator of liver metabolism and function showed steady
improvement over the first 6-12 months for those patients surviving
beyond 6 months. However, a decrease in cholinesterase near the
end of the study was also noted. These fluctuating laboratory values
lead to some speculation that the implanted cells may in some cases not have been great enough in number, or may not have maintained
viability or established meaningful functionality, sufficient to allow
long-term supplementation of liver function. Alternatively, these
fluctuations may be a natural long-term development of natural
disease progression. It is expected that implants would not attain any
level of functionality before 6 weeks post-implantation as the lack
of vascularization limits the activity of the implanted cells. Prior to
vascularization, the implants rely exclusively on diffused oxygen and
nutrients. We can speculate that the implantation and vascularization
processes may be expedited through the use of exogenous angiogenesis
factors, and suggest that this be addressed in future studies.
Finally, according to the Karnofsky scale [17], most patients
did experience a general improvement in condition (Figure 8). It is
still not certain if this is related to the implanted cells or to natural
improvement of the cirrhosis itself because the patients were still
under conservative standard treatment for cirrhosis.
All patients in this study had limited potential for survival
without liver transplant, and it is important to note that at no time
were patients involved in this study prevented from receiving a liver
transplant as a “rescue therapy by transplant”. In fact, the procedure
could be used as a bridge to transplant in selected patients.
However, at the time of the study there was no coordinated
liver transplant program of any note in Indonesia, therefore the
probability of a suitable donor being found was extremely low. In
contrast, the German study either removed or maintained patients
on the transplant list as there is a more effective transplant program in Germany [14].
Limitations
A number of limitations of this phase I study are noted. Importantly, as the study was primarily designed to assess safety, it was not sufficiently powered to determine significant clinical efficacy. Furthermore, placebo control arm in this trial would not have been ethically acceptable. Many physicians set a viral load limit of 104 iU/ mL to define “inactive” hepatitis. We included patients with up to 3 × 105 and although we didn’t detect any correlation between the viral load and patient outcome, we feel that future studies should apply a lower cut-off, as this is more likely to aid effective patient recovery. We acknowledge that cell viability and number of seeded cells with respect to the production of the tissue matrix are not discussed in this manuscript; they are the subject of a separate manuscript, as we focus on the clinical aspects of the technique in the current discussion. The single-center setting of this study might also be considered a limitation. This study was originally planned, and received all relevant ethical approvals, to be carried out in parallel in Indonesia and in Zurich, Switzerland. Unfortunately, due to logistical considerations we were unable to begin the study arm based in Switzerland.
Conclusion
We have shown for the first time in an ethically-approved clinical
study in humans that the transplantation of autologous hepatocyte
tissue matrices for the treatment of end stage liver cirrhosis is a
generally safe procedure, provided that patients are appropriately
selected and monitored. Although the necessarily small size of our
study limits our ability to show clear clinical efficacy, we do observe
clinically relevant improvements in a number of clinical and laboratory
indicators of liver function such as albumin and cholinesterase for
some patients. Positive benefits were also seen in other measures for
some patients with improvements in stamina, endurance to physical
stress, frequency of hospitalization, incidence of encephalopathy, GI
bleeding, ascites, and oesophageal varices.
Overall, we believe that the safety and clinical outcomes of this
study represent sufficient data to pursue a phase II follow-up study
that could prove the efficacy of this autologous cell transplantation
technique more definitively. Stricter inclusion and exclusion criteria
and a precisely defined and implemented follow-up protocols for
the management of possible clinical risk factors and complications
will greatly benefit the next phase of trials, as would more patients to
provide greater statistical power.
Finally, recent advances in tissue matrix generation and coating
technology have resulted in new matrix formulations that are
expected to greatly improve autologous cell seeding and culturing
and to enhance the clinical viability of implanted tissue.
References
- Soltys KA, Soto-Gutierrez A, Nagaya M, Baskin KM, Deutsch M, Ito R, et al. Barriers to the successful treatment of liver disease by hepatocyte transplantation. J hepatol. 2010;53(4):769-74.
- Bilir BM, Guinette D, Karrer F, Kumpe DA, Krysl J, Stephens J, et al. Hepatocyte transplantation in acute liver failure. Liver transpl. 2000;6(1):32-40.
- Fox IJ, Chowdhury JR. Hepatocyte transplantation. Am J Transplant. 2004;4:7-13.
- Ito M, Nagata H, Miyakawa S, Fox IJ. Review of hepatocyte transplantation. Journal of hepato-biliary-pancreatic surgery. 2009;16(2):97-100.
- Timm F, Vollmar B. Heterogeneity of the intrahepatic portal venous blood flow: impact on hepatocyte transplantation. Microvasc Res. 2013;86:34-41.
- Koenig S, Stoesser C, Krause P, Becker H, Markus PM. Liver repopulation after hepatocellular transplantation: integration and interaction of transplanted hepatocytes in the host. Cell Transplant. 2005;14(1):31-40.
- Hughes RD, Mitry RR, Dhawan A. Hepatocyte transplantation for metabolic liver disease: UK experience. JRSM. 2005;98(8):341-5.
- Vacanti JP, Morse MA, Saltzman WM, Domb AJ, Perez-Atayde A, Langer R. Selective cell transplantation using bioabsorbable artificial polymers as matrices. Journal of pediatric surgery. 1988;23(1):3-9.
- Chen G, Okamura A, Sugiyama K, Wozniak MJ, Kawazoe N, Sato S, et al. Surface modification of porous scaffolds with nanothick collagen layer by centrifugation and freeze-drying. J biomed mater res Part B Appl biomater. 2009;90(2):864-72.
- Kaufmann PM, Kneser U, Fiegel HC, Pollok JM, Kluth D, Izbicki JR, et al. Is there an optimal concentration of cotransplanted islets of Langerhans for stimulation of hepatocytes in three dimensional matrices? Transplantation. 1999;68(2):272-9.
- Uyama S, Kaufmann PM, Takeda T, Vacanti JP. Delivery of whole liver-equivalent hepatocyte mass using polymer devices and hepatotrophic stimulation. Transplant. 1993;55(4):932-5.
- Johnson LB, Aiken J, Mooney D, Schloo BL, Griffith-Cima L, Langer R, et al. The mesentery as a laminated vascular bed for hepatocyte transplantation. Cell Transplant. 1994;3(4):273-81.
- Fitzpatrick E, Mitry RR, Dhawan A. Human hepatocyte transplantation: state of the art. J Internal Med. 2009;266(4):339-57.
- Schwarz A, Lindl T, Höhneke C, Stange M, Pieken W. Human Autologous Liver Cell Transplantation for the Treatment of Cirrhosis. Internet J Gastroenterol. 2009;10(1).
- Liao CJ, Chen CF, Chen JH, Chiang SF, Lin YJ, Chang KY. Fabrication of porous biodegradable polymer scaffolds using a solvent merging/particulate leaching method. J Biomed Materials Res. 2002;59(4):676-81.
- Friedman LS. Surgery in the patient with liver disease. Trans Am Clin Climatol Assoc. 2010;121:192-205.
- Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: CM M, editor. Evaluation of chemotherapeutic agents. New York: Columbia University Press; 1949;196.