Research Article
Design and Validation of a Novel and Cost-Effective Animal Tissue Model for Training Laparoscopic Adhesiolysis and Mesh Repair of an Incisional Hernia
Porter DJ*, Ross G, Yung D, Payne C and Tang B
Department of General Surgery, University of Dundee, UK
*Corresponding author: Porter DJ, Department of General Surgery, University of Dundee, Ninewells Hospital and Medical School, Cuschieri Skills Centre, Dundee, Scotland, UK
Published: 20 Sep, 2018
Cite this article as: Porter DJ, Ross G, Yung D, Payne
C, Tang B. Design and Validation of
a Novel and Cost-Effective Animal
Tissue Model for Training Laparoscopic
Adhesiolysis and Mesh Repair of an
Incisional Hernia. World J Surg Surgical
Res. 2018; 1: 1062.
Abstract
Objectives: To design and validate a new and cost-effective animal tissue training model for
practicing laparoscopic adhesiolysis and mesh repair of an incisional hernia.
Methods and Materials: A laparoscopic training box is mounted with neoprene, which simulates
the anterior abdominal wall. The greater curve of a porcine stomach is dissected out and a small
circular defect is cut out of the double-layered stomach, this represents the hernial defect. Porcine
omentum is stapled around the defect in the stomach, and this represents the adhesions around the
incisional hernia.
A Prolene mesh is inserted into the simulated peritoneal cavity under laparoscopic vision and
tacked to the anterior abdominal wall.
Face, content, and construct validity of the model was carried out using a 5-point Likert scale
questionnaire, and comparison in task performance between course delegates and experts was made
using observational and clinical human reliability analysis.
Results: A total of 33 course delegates and 8 expert surgeons were recruited to this study from June
2016 to June 2017. Of the 33 course delegates, 24 were male and 9 were female, with age ranges
27-48 years. Course delegates had between 2 years and 9 years of laparoscopic experience. The
mean score on specific feature of the anatomy and colour, sensation of texture, maintenance of
pneumoperitoneum and adhesiolysis and mesh fixation was 4.12 ± 0.78, 4.00 ± 0.79, 4.73 ± 0.52,
4.12 ± 0.78 and 4.61 ± 0.56 respectively in the course delegates group, and 4.38 ± 0.52, 4.25 ± 0.71,
4.25 ± 0.71, 4.75 ± 0.46 and 4.75 ± 0.46 respectively in the expert surgeon group on a scale of 1
(unrealistic) and 5 (very realistic).
Conclusion: A newly designed restructured animal tissue model for training laparoscopic
adhesiolysis and mesh repair of an incisional hernia is reported. Validation studies on the model
demonstrate that this is a very realistic and effective model for skills training in laparoscopic
adhesiolysis and mesh repair of an incisional hernia.
As a result of this laparoscopic training model course delegates reported that they had gained
transferrable operating skills and increased confidence in the performance of laparoscopic
adhesiolysis and incisional hernia repair with mesh.
This training model is an effective and cost-efficient laparoscopic training simulator.
Keywords: Animal tissue model; Simulation training; Training model validity; Laparoscopic
adhesiolysis; Incisional hernia repair
Competences
Practice-based Learning and Development; Medical knowledge; Patient care; Simulated surgical training.
Introduction
Laparoscopy has become the standard approach for many conditions in most surgical specialities
[1]. This development has been driven by the desire for less surgical trauma, faster post-operative
recovery, shorter hospital stay, and better cosmetic results [2].
It is evident however that laparoscopic surgery is associated with
a longer operating time and a higher rate of surgical complications
during the learning curve of the trainee surgeon. This has been verified
in many studies within the surgical sub-specialities [3-6]. However,
the reduction of working hours introduced by the European Working
Time Directive (EWTD) and the dissolution of the traditional ‘firm’
structure have significantly reduced surgical training time and
mentorship making it difficult for trainees to perform a sufficient
number of procedures to attain competence in these complex
procedures to achieve safe and independent practice [7]. The technical
skills required for laparoscopic surgery are fundamentally different
from those for traditional open surgery, leading to a prolonged
learning curve. The primary obstacles in learning laparoscopic surgery
are psychomotor and perceptual. The unique nature of laparoscopic
surgery combined with an increasing focus on patients’ safety
and rights, the present reduction in working hours, and concerns
over costs of operating theatre time are factors that challenge the
traditional surgical approach and contribute to a growing need for
novel methods in the training of laparoscopic surgeons [8]. Virtual
reality simulation has the potential to offer important advantages in
the area of training for new skills and procedures. Perhaps one of the
most compelling driving forces for the integration of simulation into
surgical training is the ethical imperative of providing patients with
the best care. Although it is understood that trainees will eventually
develop technical skills by treating patients, patients should not be
subjected to the possibility of harm when other training methods are
available for skills acquisition [9].
Simulation ensures that some practice has taken place before
trainees treat real patients [10]. Simulation also allows for alternative
ways to acquire skills within the constraints of working time
restrictions and reduced clinical exposure. Simulators are available at
any time to be utilized, making them flexible for training. Simulation
can also provide a means for both trainee surgeons and consultant
surgeons to acquire the necessary skills to incorporate new surgical
technologies and innovation into their surgical repertoire. In addition
simulation allows scope for error and the ability to allow trainees to
learn the consequences of error [11]. Animal training models have
been widely used for laparoscopic surgical skills training [12]. When
designing and developing such a model, the following factors should
be considered [13] (1) the model should be as realistic as possible to
simulate the anatomy and pathology involved in the procedure; (2)
skills learnt on the model can be transferred to the operating theatre;
(3) the final result of the performance can be made available for
inspection and feedback; (4) the model has the ability to distinguish
the experience of surgeons; and (5) production of the model is costeffective
and simple to allow it to be massively reproduced for a group
of participants.
A model developed has to be realistic, appropriate, and effective
as a teaching and training tool, and it should also possess the ability
to distinguish surgeons’ experience. Thus validation of reliability and
effectiveness remains critical [14-16].
Methods and Materials
Design and preparation of the restructured animal tissue model
Porcine stomach and omentum were collected from a local
abattoir that was fully registered under the standard regulations
stipulated by the meat industry and follows strict ethical guidelines.
A laparoscopic training box, a 10 mm 30 degree laparoscope, 3
ports (2 mm x 11 mm and 1 mm x 5 mm), a Prolene mesh, a 1-0 Vicryl
suture, a ruler and a marker pen and chalk, a laparoscopic tacking
device, 2 x laparoscopic graspers, hook diathermy or a laparoscopic
Harmonic scalpel, 4 x mosquito forceps, a 10 mm syringe, normal
saline, a needle, a laparoscopic stack, laparoscopic and external
scissors and a suture retriever are required to perform the simulated
laparoscopic adhesiolysis and mesh repair of an incisional hernia.
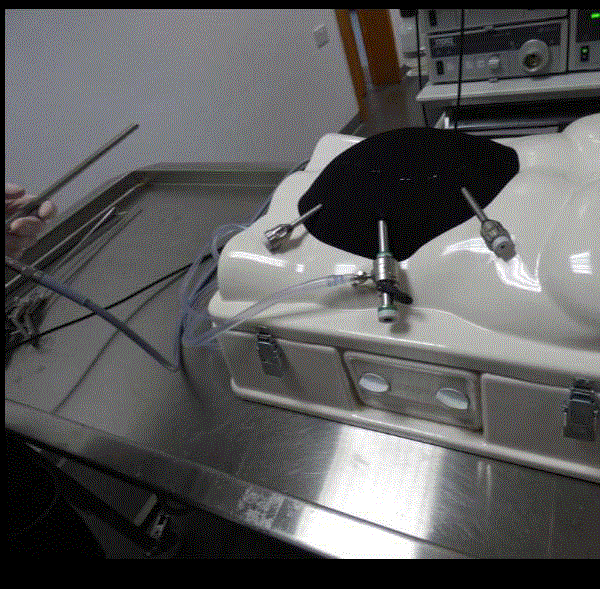
The laparoscopic training box was mounted with neoprene,
which simulates the anterior abdominal wall. The neoprene on the
laparoscopic trainer creates an air tight seal and therefore maintains
a pneumoperitoneum when created (Figure 1). The greater curve of a
porcine stomach is dissected out and a small circular defect is cut out
of one layer of the double-layered stomach, this represents the hernial
defect. Porcine omentum is stapled around the defect in the stomach,
and this replicates the adhesions around the incisional hernia.
An experienced consultant general and colorectal surgeon
provided close supervision and instruction during the construction
of the simulated model.
The cost of making a complete laparoscopic simulation model
was approximately £50, which included labour and materials.
Use of the animal tissue model for laparoscopic
adhesiolysis and mesh repair of an incisional hernia
Course delegates insert an 11 mm laparoscopic port and gain
pneumoperitoneum in a standard fashion. Diagnostic laparoscopy
is then performed and the other 11 mm port and the 5mm port
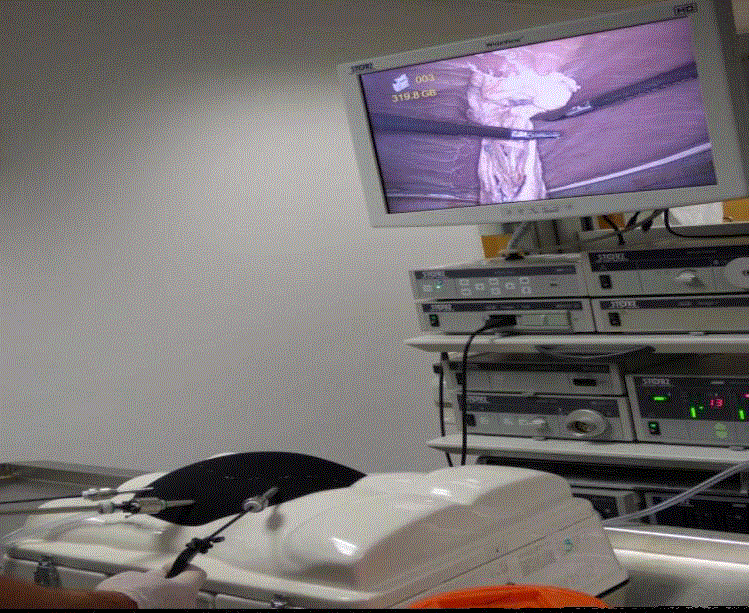
are inserted under direct vision. A laparoscopic adhesiolysis is
undertaken where the omental adhesions around the hernia defect
are taken down using hook diathermy, the Harmonic scalpel or
laparoscopic scissors (Figure 2).
When the omental adhesions have been removed, the hernia
defect becomes apparent. The diameter of the defect is measured with
the ruler and its dimensions marked externally with the chalk, before
cutting a piece of prosthetic mesh to size, using the ruler and marker
pen for accuracy. Four stay sutures are inserted into the corners of the
mesh with the 1-0 Vicryl suture.
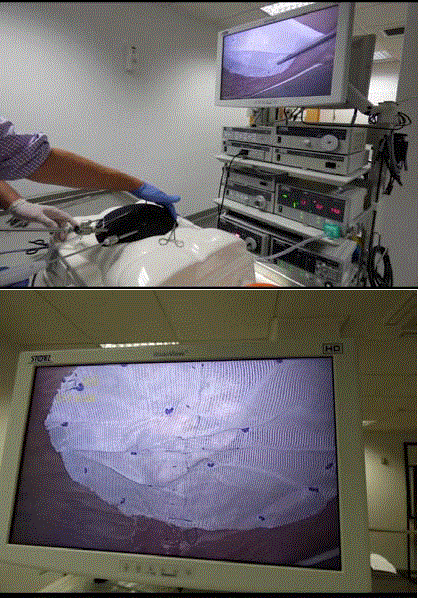
The mesh is then rolled and inserted via the 11 mm port and
unrolled within the simulated peritoneal cavity under laparoscopic
vision. The stay sutures are used to approximate the mesh to the
anterior abdominal wall internally, drawn out through the abdomen
using the suture retriever and held in place using the mosquito
forceps. The mesh is then tacked to the anterior abdominal wall using
the laparoscopic tacking device (Figures 3 and 4).
Transverse abdominis plane (TAP) blocks are then sited under
laparoscopic vision, and the ports are removed under vision.
Face and content validity
A clear announcement of voluntary participation was made to the
course delegates, and who gave consent before participating in the
study.
Criteria for validity were defined based on the definition and
recommendation that are commonly used for validity testing for
laparoscopic models and simulators [14-16].
Face validity relates to the degree of realism of the simulator
in relation to the real anatomy and setup, whereas content validity
involves the measurement of the appropriateness of the simulator as
an effective modality [14].
A structured questionnaire was designed for face and content
validity of the laparoscopic simulator based on subjective assessment
by both participants and experts.
At the end of the course, all participants and experts completed
this questionnaire to assess the validity of the model for laparoscopic
adhesiolysis and hernia repair training [13].
The evaluation of realism on (1) anatomy and colour, (2)
sensation of texture and feeling of dissection of the tissues, (3) efficacy
and safety of the skills, (4) maintenance of pneumoperitoneum, (5)
laparoscopic adhesiolysis, (6) mesh handling and fixation, and (7)
fixation of the mesh to the abdominal wall were the end points for
assessment of the face validity of the adhesiolysis and hernia repair
model (Table 1).
Questions such as ‘Is this a useful model for training in
laparoscopic adhesiolysis and hernia repair? ‘Do you think the skills
learnt from this model are transferrable to the operating theatre?’ ‘Do
you feel more confident in performing laparoscopic adhesiolysis and
incisional hernia repair after practicing on this model?’ ‘Do you think
this model can be used as a routine training model for lap incisional
repair?’ were used for content validity.
Data collection and statistical analysis
Data was collected using a Likert scale (1 = strongly disagree; 2 =
disagree; 3 = neither agree nor disagree; 4 = agree; and 5 = strongly
agree) on a standardized anonymous questionnaire. Both course
delegates and expert surgeons completed evaluation forms, and
analysis of the feedback was performed. Excel (Microsoft Office 2013)
and statistical Package for the Social Sciences version 16 were used for
data collection and analysis.
Figure 1
Figure 2
Figure 3 and 4
Figure 3 and 4
The mesh is then tacked to the anterior abdominal wall
using the laparoscopic tacking device.
Table 1
Results
Demographics of participants and experts
A total of 33 course delegates were recruited to this study from
June 2016 to June 2017. Twenty four of the course delegates were
male and 9 were female, with ages ranging from 27 to 48 years. Course
delegates had between 2 and 9 years of laparoscopic experience. The
expert group consisted of 7 consultant surgeons and a senior registrar
with significant experience in laparoscopic surgery, with ages ranging
from 35 to 82 years. The expert group had an average of 19.1 ± 6.90
years laparoscopic experience compared to the trainee group who
had an average of 4.55 ± 2.22 years of laparoscopic experience.
Course delegates and expert surgeons were recruited to the study
on a voluntary basis without any financial interest or other conflicts
of interest.
Outcome of face validity of the model
The overall mean satisfaction rate for the training model given
by the participants was 4.18 ± 0.77 for port position, 4.12 ± 0.78 for
anatomy and colour, 4.00 ± 0.79 for sensation and texture of the
tissue, 4.73 ± 0.52 for maintenance of pneumoperitoneum, 4.12 ± 0.78
for laparoscopic adhesiolysis and 4.61 ± 0.56 for mesh handling and fixation on a scale of 1 (unrealistic/poor) to 5 (very realistic/useful),
whereas the experts rated these parameters as 4.50 ± 0.53, 4.38 ± 0.52,
4.25 ± 0.71, 4.25 ± 0.71, 4.75 ± 0.46 and 4.75 ± 0.46 respectively (Table
1).
Content validity
Both participants and experts agreed that this was a very useful
and effective model for training in laparoscopic adhesiolysis and mesh
repair of an incisional hernia (4.79 ± 0.42 vs. 4.63 ± 0.52 respectively
(Table 1). The trainees felt that the skills acquired from this model
could be transferred to the operating theatre.
Both course delegates and experts felt that this model could be
used as a routine training model for laparoscopic adhesiolysis and
mesh repair of an incisional hernia (4.67 ± 0.48 vs. 5.00 ± 0 (Table 1).
Discussion
Virtual reality simulators for laparoscopic surgical skills training
are an invaluable training resource [13]. However animal tissue
models have been shown to be superior and are the preferred method
for surgical trainees to acquire technical skills in laparoscopic surgery
when a suitable organ or tissue can be found in an animal [12].
When suitable and realistic anatomy cannot be found naturally, a
restructured animal tissue model can become a valuable and effective
training resource.
Restructured animal tissue models have been successfully
developed and used in a variety of different laparoscopic procedures
such as laparoscopic salpingectomy and laparoscopic fundoplication
in gynaecological and general surgical training respectively [17,18].
These models have been proven to be realistic, cost-effective, and
simple enough to be produced for use in laboratory-based surgical
training courses with a large number of surgical trainees [17,18].
In addition when these restructured animal tissue models are used
during surgical training courses the final results of the procedures can
be assessed, feedback can be given to the trainees, and the exercise can
be repeated [17,18].
The materials and methods used to develop this laparoscopic
adhesiolysis and incisional hernia repair model with restructured
porcine stomach and greater omentum within a laparoscopic training
simulating box have been described in detail in this article. The key
features that were considered when designing and developing this
model were realistic anatomy, effective simulation repetitive practice,
feedback on performance, simulator validity, and cost [13].
The aim of this article was that by describing the details of the
materials used and methods applied to design and develop this
simulated training model, that surgical trainers could replicate this
model and integrate it into their training program [19].
There is no doubt that when compared to synthetic training
models, animal training models and virtual reality simulators,
human cadavers remain the most realistic training model for many
laparoscopic and open surgical procedures. It is our recommendation
that junior and intermediate surgical trainees acquire basic and
intermediate level laparoscopic skills on a restructured animal tissue
model before progressing to simulation training on cadavers when
they attend advanced laparoscopic training courses.
It is essential to validate a simulator to examine its fidelity,
authenticity, and efficiency before it is integrated into a training
course [13-16,20-23]. Therefore the face and content validation of
this laparoscopic training model was performed.
Expert surgeons found port position, anatomy and colour of
the model, sensation and texture of the tissues, efficacy and safety
of the skills exercise and mesh handling more realistic than the
trainee surgeons but this difference was not statistically significant.
However expert surgeons found laparoscopic adhesiolysis on the
model more realistic than the course delegates and this difference was
statistically significant. This is a positive endorsement of this training
tool as expert surgeons with vast experience in laparoscopic surgery
found the model realistic and skills acquired during this exercise
transferrable to real-life surgery. Expert surgeons found maintenance of pneumoperitoneum and fixation of mesh to the abdominal wall
less realistic than the trainee surgeons and these differences were
statistically significant (Table 1).
In terms of content validity trainees rated the model more
useful for teaching laparoscopic incisional hernia repair than expert
surgeons but this difference was not statistically significant. Both
trainees and expert surgeons felt that skills acquired with this training
tool were highly transferrable and increased confidence was gained
in laparoscopic skills as a result of using this training tool, and all
agreed that this training model should be integrated into laparoscopic
training.
Compared with the other existing training models, the major
advantages of this laparoscopic adhesiolysis and mesh repair of an
incisional hernia model are: 1) real animal tissue was used; hence
tissue planes could be appreciated, tissue handling was on par with
real - life laparoscopic surgery, and haptic feedback was present;
2) the relevant anatomy and pathology in the training tool was
restructured as closely as possible to real-life anatomy and pathology;
3) real electrosurgery, real equipment and instruments were utilized
during the exercise; 4) the final results of the laparoscopic training
tool were validated over a one year period with course delegates and
independent expert surgeons, and 5) the model was cost effective
[17]. The model cost £50 and hence the cost was minimal compared
to other simulators [13,17,22]. Despite the high scores achieved from
both expert surgeons and trainee surgeons, the major disadvantage
of this model is that it did not simulate bleeding that is an essential
skill to learn to avoid and manage when it occurs. A potential future
improvement in this training model might be to simulate intraoperative
bleeding so that the model is more applicable to real life.
There was also a lack of objective data to demonstrate whether skills
learned on this model could be transferred to improved performance
in the operating theatre (criterion validity). Further studies on
criterion validity should be conducted if this model is to be used as an
assessment tool for the trainees in the future.
Conclusion
A newly designed restructured animal tissue model for training
laparoscopic adhesiolysis and mesh repair of an incisional hernia is
reported. Validation studies on the model demonstrate that this is
a very realistic and effective model for skills training in laparoscopic
adhesiolysis and mesh repair of an incisional hernia.
As a result of this laparoscopic training model course delegates
reported that they had gained transferrable operating skills and
increased confidence in the performance of laparoscopic adhesiolysis
and incisional hernia repair with mesh. This training model is an
effective and cost-efficient laparoscopic training simulator.
References
- Keus F, Broeders IA, van Laarhoven CJ. Gallstone disease: surgical aspects of symptomatic cholecystolithiasis and acute cholecystitis. Best Pract Res Clin Gastroenterol. 2006;20:1031-51.
- Larsen CR, Soerensen JL, Grantcharov TP, Dalsgaard T, Schouenbourg L, Ottosen C, et al. Effect of Virtual Reality Training On Laparoscopic Surgery: Randomised Controlled Trial. BMJ. 2009:338:b1802.
- Karvonen J, Gullichsen R, Laine S, Salminen P, Gronroos JM. Bile duct injuries during laparoscopic cholecystectomy: primary and long-term results from a single institution. Surg Endosc. 2007;21(7):1069-73.
- Avital S, Hermon H, Greenberg R, Karin E, Skornick Y. Learning curve in laparoscopic colorectal surgery: our first 100 patients. Isr Med Assoc J. 2006;8(10):683-6.
- Kumar U, Gill IS. Learning curve in human laparoscopic surgery. Curr Urol Rep. 2006;7(2):120-4.
- Eto M, Harano M, Koga H, Tanaka M, Naito S. Clinical outcomes and learning curve of a laparoscopic adrenalectomy in 103 consecutive cases at a single institute. Int J Urol. 2006;13(6):671-6.
- Reznick RK, MacRae H. Teaching surgical skills-changes in the wind. N Engl J Med. 2006;355(25):2664-9.
- Grantcharov TP, Reznick RK. Teaching procedural skills. BMJ. 2008;336(7653):1129-31.
- Ziv A, Wolpe PR, Small SD, Glick S. Simulation-based medical education: an ethical imperative. Acad Med. 2003;78(8):783-8.
- Issenberg SB, McGaghie WC, Hart IR, Mayer JW, Felner JM, Petrusa ER, et al. Simulation technology for health care professional skills training and assessment. JAMA 1999;282(9):861-6.
- Maran NJ, Glavin RJ. Low to high fidelity simulation-a continuum of medical education? Med Educ. 2003;37(Suppl 1):22-8.
- Van Bruwaena S, Schijven MP, Napolitano D, De Win G, Miserez M. Porcine cadaver organ or virtual reality simulation training for laparoscopic cholecystectomy: a randomized-controller trial. J Surg Educ. 2015;72(3):483-90.
- Issenberg SB, McGaghie WC, Petrusa ER, Lee Gordon D, Scalese RJ. Features and Uses of high-fidelity medical simulators that lead to effective learning: a BEME systematic review. Med Teach. 2005;27(1):10-28.
- McGougall EM. Validation of Surgical Simulators. J Endourol. 2007;21(3):244-7.
- Van Nortwick SS, Lendvay T, Jensen A, Wright AS, Horvath KD, Kim S. Methodologies for establishing validity in surgical simulation studies. Surgery. 2010;147(5):622-630.
- Ayodeji ID, Schijven M, Jakimowicz J, Greve JW. Face Validation of the Simbionix LAP Mentor Virtual reality training module and its applicability in the surgical curriculum. Surg Endosc. 2007;21(9):1641-9.
- Tang B, Tait I, Ross G, Chien P. Development and use of a restructured animal tissue model for training laparoscopic salpingectomy and salpingectomy. J Minim Invasive Gynecol. 2011;18(6):785-91.
- Carter F, Russell E, Dunkley P, Cuschieri A. Restructured animal tissue model for training in laparoscopic anti-reflux surgery. Minim Invasive Ther Allied Technol. 1994;3(2):77-80.
- Khan MS, Ahmed K, Gavazzi A, Gohil R, Thomas L, Poulsen J, et al. Development and implementation of centralized simulation training: evaluation of feasibility, acceptability and construct validity. BJU Int. 2013;111(3):518-23.
- Bright E, Vine S, Wilson MR, Masters RSW, McGrath J. Face validity, construct validity and training benefit of a virtual reality TURP simulator. Int J Surg. 2012;10(3):163-6.
- Brewin J, Ahmed K, Khan M, Jaye P, Dasgupta P. Face, content, and construct validation of the Bristol TURP trainer. J Surg Edu. 2014;71(4):500-5.
- Sweet R, Kowalewski T, Oppenheimer P, Weghorst S, Satava R. Face, content and construct validity of the University of Washington virtual reality transurethral prostate resection trainer. J Urol. 2004;172(5):1953-7.
- Schout BM, Hendrikx AJ, Scheele F, Bemelmans BL, Scherpbier AJ. Validation and implementation of surgical simulators: a critical review of present, past and future. Surg Endosc. 2010;24(3):536-46.