Review Article
Pseudoangiomatous Stromal Hyperplasia (PASH) in Adolescence: A Systematic Review
Dottoressa Francesca Pellini1*, Maya Lorenzi1, Rossella Gaudino2, Beatrice Accordini1, Sara
Mirandola1, Alessandra Invento1 and Giovanni Paolo Pollini1
1Department of Breast Unit Surgery, Azienda Ospedaliera Integrata di Verona, Italy
2Department of Surgery, Dentistry, Paediatrics and Gynaecology, University of Verona, Italy
*Corresponding author: Dottoressa Francesca Pellini, Department of Breast Unit Surgery, Azienda Ospedaliera Integrata di Verona, Piazzale Aristide Stefani, 1, Verona VR, Italy
Published: 17 Sep, 2018
Cite this article as: Pellini DF, Lorenzi M, Gaudino R,
Accordini B, Mirandola S, Invento
A,et al. Pseudoangiomatous Stromal
Hyperplasia (PASH) in Adolescence:
A Systematic Review. World J Surg
Surgical Res. 2018; 1: 1058.
Abstract
Objective: Pseudoangiomatous Stromal Hyperplasia (PASH) of the breast is a benign mesenchymal
proliferative lesion occurring most commonly in middle-aged, premenopausal women and it’s
exceptionally rare in adolescents. The aim of this study is to present a review of the literature on
PASH in childhood, comparing its results with our new cases, and to define a standard approach
for its treatment.
Design and Patients: We describe PASH in 3 pediatric patients and compare them with 24
adolescent cases described in literature.
Main Outcome Measures: Primary outcome was the type of treatment in adolescent patients with
diagnosis of breast PASH. Secondary outcomes were the evidence of recurrence and the cosmetic
results, associated with the patient satisfaction.
Results: All 24 patients described in literature underwent surgical excision of the lesion, the large
majority a breast-conserving procedure. None of them had a recurrence, except for an adolescent
female. Our 3 patients underwent surgically excision through breast-conserving circumareolar
incisions. A slight breast asymmetry was still remaining, but it is improving spontaneously with the
patient’s growing.
Conclusion: PASH benign nature and complete healing after surgical resection represent aspects
of tranquility which must be communicated to the patient, often worried because of the big size
and the fast growth of the lesion. Preoperative core biopsy and hormonal therapy could be two new
instruments to avoid surgery in some patients, but more likely in adults.
Keywords: PASH; Adolescence; CD31; CD34
Introduction
Pseudoangiomatous Stromal Hyperplasia (PASH) is a rare benign mesenchymal proliferative
lesion of the breast. Since its first description by Vuitch, Erlandson and Rosen in 1986, about 200
cases had been documented in the literature [1], including only around 20 adolescents. It occurs
most commonly in middle-aged, premenopausal women; the age of the diagnosis varies between 14
to 74 years, but it’s exceptionally rare in adolescents [2,3].
PASH is a clinical entity now well-known, but which still eludes knowledge of biological
characteristics of the tumor. Breast tissue affected by PASH is characterized by dense myofibroblastic
proliferation of mammary stroma, associated with inter anastomosing capillary-like space. Such
morphology is the basis of the name of the lesion: Leon et al. proposed the term myofibroblastic
hyperplasia of the mammary stroma to denote its true histogenesis. More recently it has been
proposed to rename the injury to put greater emphasis on the characteristic type of cell PASH
(Figure 1).
The exact etiology and pathogenesis of PASH is still unknown, but there are much evidence
showing that the basis of the development of PASH is a prolonged hormonal (primarily
progestogenic) stimulus. In general, it is believed to be an aberrant reactivity of myofibroblasts to
endogenous or exogenous hormones. This strong hormonal component is supported by the fact
that PASH appears most commonly in premenopausal women or in older women taking estrogen
replacement. PASH is very similar histologically to the normal mammary stroma during the luteal phase of the menstrual cycle.
More often, PASH clinically presents as a firm, painless and
movable single mass, with no associated nipple or skin changes, but
it can infrequently be diffuse or multinodular. The size of PASH
usually ranges between 0.6 cm to 12 cm with most cases ranging from
small to medium size. It may present in a wide clinicopathologic
spectrum, ranging from incidental focal microscopic findings to
clinically symptomatic and mammographically evident breast masses
[4]. In young patients it usually presents as a fast-growing palpable
lesion; this may be attributed to the hormonal milieu of puberty and
adolescence [5,6].
Mammography of breast masses arising from PASH reveals a
discrete, dense homogenous lesion lacking calcifications, however,
mammography has had limited application in adolescence because
of the more fibrotic nature of the breast tissue, which may either
obscure identification of lesions or lead some to interpret normal
development as possible suspicious lesions.
Unfortunately, neither the ultrasound, nor the RM is specific
enough to allow a definitive diagnosis to be obtained. The cytology
also rarely provides a diagnosis, thus a histological examination
is necessary. On gross examination, PASH commonly occurs
as a sharply circumscribed and well encapsulated breast lesion,
occasionally presenting in a diffuse form. Typically, the cut surface is
smooth, firm or rubbery. It has typically a glistering surface and varies
in color from gray to tan-pink, yellow or white.
Breast lesions are uncommon in children and adolescents. The
most common masses are benign tumors like fibroadenomas or are
associated with inflammation due to infection [7]. Among breast
masses in adolescent females, some pathologic lesions such as giant
fibroadenoma, phyllodes tumor, PASH, juvenile papillomatosis
(Swiss cheese disease) and virginal breast hypertrophy (juvenile
macromastia) rapidly and massively increase in size over a short time
period. Other less common causes are lipoma, mammary hamartoma,
breast abscess, fibrocystic change and adenocarcinoma.
The differential diagnosis of a large breast mass in adolescent
females is important for determine treatment modalities. PASH
may grow quickly and often is mistaken for fibroadenoma or
phyllodes tumors, but the most important differential diagnosis
on histopathological examination is low-grade angiosarcoma.
Angiosarcoma is characterized by interanastomosing vascular
channels with invasion into the breast parenchyma, papillary
endothelial growth and hyperchromatic endothelial cells. In
problematic cases immunohistochemistry can be helpful.
Immunohistochemical staining of PASH expresses CD34,
vimentin and at least focally smooth muscle actin, desmin and bcl-2,
but not endothelial markers (CD31, Factor VIII), S100 or cytokeratin
[8,9].
Treatment strategies for PASH remain controversial. Wide
surgical resection or mastectomy is requested when there is an
important mass-effect by PASH, whereas other cases may only
require local excision or conservative therapy. However, some cases
with diffuse involvement or multiple recurrences may necessitate
mastectomy to achieve complete resection, while in others close
interval follow-up with careful clinical and imaging correlation could
be acceptable, rather than surgical excision [10]. Importantly, even
though it is benign, PASH has a tendency to recur if incompletely
excised, so it must be resected with careful attention to resection
around the capsule of the tumor with breast conservation as a goal.
Regardless of a benign origin of the lesion and good prognosis,
long-term follow-up is recommended for all patients, as some have
been reported to recur.
We describe PASH in 3 pediatric patients and compare them with
24 adolescent cases described in literature.
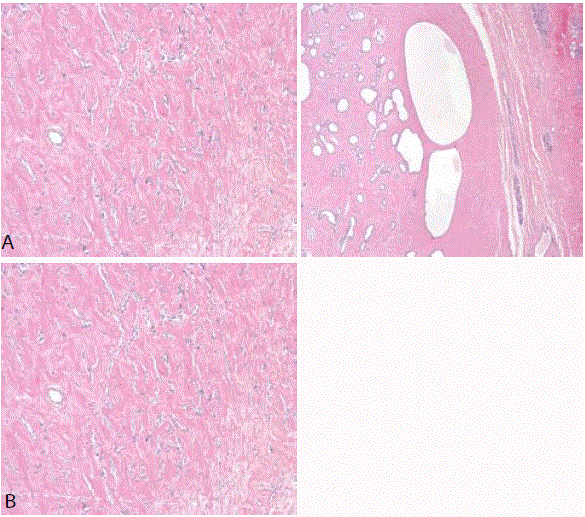
Figure 1
Figure (1A-1B)
Microscopic aspects of the nodular area: stromal fibrosis
with proliferation of myofibroblasts in an anomalous pattern that resembles
vascular spaces.
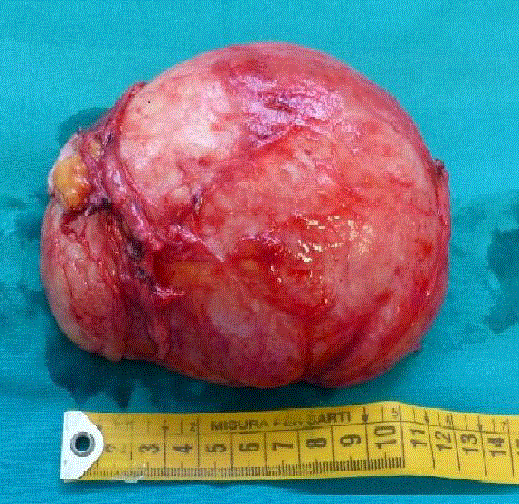
Figure 2
Figure 2
Macroscopic tumor appearance: 12 cm × 10 cm × 6 cm in diameter,
homogeneous appearance with a smooth whitish-gray surface, weighing
395g.
Methods and Materials
We performed a systematic search of the PubMed database from inception to June 2018 using database-specific syntaxes of keywords relevant to ‘pseudoangiomatous stromal hyperplasia’, ‘childhood’ and ‘adolescent’. We then compared these results with that one from a retrospective review of our institution’s surgical pathology database for the histologic diagnosis of PASH from January 2000 through April 2018 among patients between 10 and 18 years of age. Patients’ medical records were retrospectively reviewed for information regarding the patient’s demographics, personal and family history of cancer, presentation, clinical, imaging and pathological diagnoses and treatment. Clinical follow-up, imaging and pathological findings were also recorded, where available. All patients’ identifiers were kept confidential.
Results
Only 24 cases of PASH in adolescent patients are described in
literature until 2018. The study published by Shehata et al. in 2009
presented the largest number of pediatric patients with PASH,
including 9 females and 3 males [11]. Other studies are all case reports
describing one or two young female patients.
Almost all of these patients went to breast clinics because of a
fast growing breast mass causing them breast asymmetry. Frequently
there were no other associated symptoms and only 2 of the 24
patients [12,13] had a painful mass. First of all, clinical examinations
always revealed rapidly growing and mobile masses, with no
lymphadenopathy, and after that all patients underwent bilateral
breast ultrasonography: lesions appeared often well-defined, hypoechoic
and slightly heterogeneous, but the results were not specific.
Mammography is only once described [10], probably because of
its low sensitivity in young people: it showed a likely benign mass
with asymmetric margins that tailed off into the surrounding breast
tissue without microcalcifications. RMN was performed in 2 of
the 24 patients for staging the breast tumor. Core-biopsy had been
performed and permitted the diagnosis before surgery in 6 cases,
while 18 of the 24 patients were diagnosed on surgical excision of the
breast mass.
All 24 patients underwent surgical excision of the lesion; the large
majority had a breast-conserving procedure. A few patients, instead,
underwent a mastectomy: two because of a gynecomastia associated
[11,14] and two because of a markedly enlarged breast [15]. Five
patients presented with breast asymmetry with masses in both breast
and needed bilateral surgery [11,13,15,16].
Conservative surgery needs healthy breast tissue margins to avoid
recurrence. None of the 24 patients had a recurrence of their PASH
after surgical excision, except for an adolescent female, described
in Singh case report, which required bilateral mastectomies after
surgical excisions secondary to PASH recurrence before the age of 13.
We report 3 cases of adolescent girls who presented in our breast
clinic between 2010 and 2018 with unilateral breast PASH. The
clinical history of all them was similar and not significant; they were
in good general health. They presented to the breast clinic, sent by
general doctor because of a voluminous and rapidly growing breast
mass causing breast asymmetry. All cases occurred after menarche
with regular menses, normal timing of pubertal development and
were not associated alteration with hormonal imbalances. The
gonadotropin-Releasing Hormone (GnRH) stimulation test showed
values of LH and FSH in the normal pubertal range. None of the girls
took any medications, specifically no oral contraceptive or hormonal
medications; their clinical history was not significant and they were
in good general health.
Clinical examinations revealed palpable masses with elastic and
movable hard texture than the superficial and deep planes ranging in
size from 5 cm to 10 cm in diameter. Normal breast development was
evident on the opposite side. There was no axillary, supraclavicular or
lateral cervical lymphadenopathy.
The patients were submitted to bilateral breast ultrasonographic
examination that showed solid and homogeneous masses, with no
cystic component. Fine-needle aspiration cytology was performed
sonographically in one of the 3 girls and did not show cells with features
of malignancy, but the result was nonspecific and inconclusive; this
patient underwent also an MRI for staging of breast cancer, which
showed an 11 cm × 9 cm × 8 cm well-circumscribed mass lesions
with plateau and washout enhancement kinetics. On the T1-weighted
images the lesion was homogeneously hypointense, while on the T2-
weighted image it was heterogeneously hyperintense.
Although all these patients' findings were nonspecific, they were
suggestive of a benign process, such as fibroadenoma, Phyllodes
tumor, hamartoma or PASH. We decided not to carry out core-cut
biopsy sampling before surgical intervention to obtain a specific
differential diagnosis because surgery was anyway necessary given
the big size of the lesions and the resulting severe breast asymmetry.
Lumps were surgically excised through breast-conserving
circumareolar incisions under locoregional anaesthesia and sedation.
Histological examinations revealed oval masses with well-defined
margins, coloring to greyish (Figure 2).
A slight breast asymmetry was still remaining, but it is improving
spontaneously with the patient’s growing. The patients were happy
with the cosmetic result and enough normal breast tissue had been
preserved to enable breast development.
Patients were discharged on the day after surgery.
Histology and immunohistochemistry were diagnostic for PASH.
In particular immunohistochemical staining showed intense and
diffuse positivity of the myofibroblasts for actin and CD34 and less
than 15% of the cells were ER and PR positive in each patient.
None of the patients showed evidence of clinical or
ultrasonographic recurrence after a follow-up of at least 10 months.
Discussion
PASH is gaining acceptance as an important entity in the
differential diagnosis of adult breast lesions ever since it was first
described in 1986. While PASH is well established in adult breast
pathology, little has been reported about it in the pediatric population,
where only 24 case reports in adolescent patients exist.
PASH is frequently an incidental histologic finding in breast
biopsies performed for other reasons. Sometimes, it can present as
a firm, painless and rapidly growing breast mass, as in our patients.
Many evidences showed that the basis of the development of
PASH is a prolonged progestogenic stimulus, which can be either
endogenous or exogenous. In our patients the definitive histological
examination showed the lesion to positive PR as well as fully described
in the literature.
Our preoperative diagnostic procedures failed to identify the
nature of the lesion and the clinical suspicion addressed to a benign
fibroadenoma or borderline phyllodes tumor. It was difficult to
suspect and/or diagnose PASH also because of the rarity of PASH in
adolescence.
Recently it has been proposed by Wieman et al. to use core-biopsy
as a preoperative high sensitivity diagnostic method [17-20]. It is
essential especially if you decide to start a close follow-up or medical
treatment of the lesion instead of a surgical pathway. Sometimes PASH is diagnosed in conjunction with malignant breast lesions, so
it is necessary to ensure that the sample is sufficiently representative.
After performing biopsy, it is crucial to compare histological findings
with clinical and imaging data.
Breast-conserving surgery is the current standard of care for
PASH. The lumpectomy is technically simple thank to the solid and
well-defined structure and the recurrence rate is extremely low if the
tumor is removed with safety margins of healthy tissue. In case of
diffuse or multifocal PASH, mastectomy is required to ensure the
surgical radicality.
Recently, clinical trials have gone beyond the current standard
of care by investigating new and more conservative treatments, with
surgery only if needed. Many authors proposed short-interval followup
or medical therapy, instead of an initial surgical treatment. In
favor of this approach it has been described in the literature a case of
PASH regression after hormonal therapy with tamoxifen.
In our cases we opted for surgery given the asymmetry of the two
breasts, as well as obvious cosmetic problems, which caused a distress
in the young patients and could, generated a spoiled column posture.
Moreover, although preoperative diagnosis was not conclusive, the
rapid growth of the lesions, in contrast to the cytological findings of
mercy, forced to surgery. It is more likely that conservative approaches
will be mostly useful for adult patients in whom PASH is frequently
an incidental histologic finding in breast biopsies performed for other
reasons and not a big breast mass clinically evident.
Conclusion
PASH is a rare event in the wide spectrum of breast lesions. Its
benign nature and complete healing after surgical resection represent
aspects of tranquility which must be communicated to the patient,
often worried because of the big size and the fast growth of the lesion.
Preoperative core biopsy and hormonal therapy could be two new
instruments to avoid surgery in some patients, especially adults. New
studies are therefore desirable to determine the best approach for this
type of injury in pediatric patients.
References
- Vuitch MF, Rosen PP, Erlandson RA. Pseudoangiomatous hyperplasia of mammary stroma. Hum Pathol. 1986;17(2):185-91.
- Baker M, Chen H, Latchaw L, Memoli V, Ornvold K. Pseudoangiomatous stromal hyperplasia of the breast in a 10-year-old girl. J Pediatr Surg. 2011;46(8):e27-31.
- Taira N, Ohsumi S, Aogi K, Maeba T, Kawamura S, Nishimura R, et al. Nodular pseudoangiomatous stromal hyperplasia of mammary stroma in a case showing rapid tumor growth. Breast Cancer. 2005;12(4):331-6.
- Nassar H, Elieff MP, Kronz JD, Argani P. Pseudoangiomatous stromal hyperplasia (PASH) of the breast with foci of morphologic malignancy: a case of PASH with malignant transformation? Int J Surg Pathol. 2010;18(6):564-9.
- Virk RK, Khan A. Pseudoangiomatous stromal hyperplasia: an overview. Arch Pathol Lab Med. 2010;134(7):1070-4.
- Bowman E, Oprea G, Okoli J, Gundry K, Rizzo M, Gabram-Mendola S, et al. Pseudoangiomatous stromal hyperplasia (PASH) of the breast: a series of 24 patients. Breast J. 2012;18(3):242-7.
- Boothroyd A, Carty H. Breast masses in childhood and adolescence. A presentation of 17 cases and a review of the literature. Pediatr Radiol. 1994;24(2):81-4.
- Powell CM, Cranor ML, Rosen PP. Pseudoangiomatous stromal hyperplasia (PASH). A mammary stromal tumor with myofibroblastic differentiation. Am J Surg Pathol. 1995;19(3):270-7.
- Anderson C, Ricci AJ, Pedersen CA, Cartun RW. Immunocytochemical analysis of estrogen and progesterone receptors in benign stromal lesions of the breast. Evidence for hormonal etiology in pseudoangiomatous hyperplasia of mammary stroma. Am J Surg Pathol. 1991;15(2):145-9.
- Zubor P, Kajo K, Dussan CA, Szunyogh N, Danko J. Rapidly growing nodular pseudoangiomatous stromal hyperplasia of the breast in an 18-year-old girl. APMIS. 2006;114(5):389-92.
- Shehata BM, Fishman I, Collings MH, Wang J, Poulik JM, Ricketts RR, et al. Pseudoangiomatous stromal hyperplasia of the breast in pediatric patients: an underrecognized entity. Pediatr Dev Pathol. 2009;12(6):450-4.
- Gow KW, Mayfield JK, Lloyd D, Shehata BM. Pseudoangiomatous stromal hyperplasia of the breast in two adolescent females. Am Surg. 2004;70(7):605-8.
- Testori A, Alloisio M, Errico V, Bottoni E, Voulaz E, Fernandez B, et al. Pseudoangiomatous stromal hyperplasia - a benign and rare tumor of the breast in an adolescent: a case report. J Med Case Rep. 2017;11(1):284.
- Gallardo Munoz I, Raya Povedano JL, Santos Romero AL. [Nodular pseudoangiomatous stromal hyperplasia of the breast in two adolescents]. Radiologia. 2012;54(6):549-52.
- Singh KA, Lewis MM, Runge RL, Carlson GW. Pseudoangiomatous stromal hyperplasia. A case for bilateral mastectomy in a 12-year-old girl. Breast J. 2007;13(6):603-6.
- Teh HS, Chiang SH, Leung JW, Tan SM, Mancer JF. Rapidly enlarging tumoral pseudoangiomatous stromal hyperplasia in a 15-year-old patient: distinguishing sonographic and magnetic resonance imaging findings and correlation with histologic findings. J Ultrasound Med. 2007;26(8):1101-6.
- Wieman SM, Landercasper J, Johnson JM, Ellis RL, Wester SM, Lambert PJ, et al. Tumoral pseudoangiomatous stromal hyperplasia of the breast. Am Surg. 2008;74(12):1211-4.
- Salvador R, Lirola JL, Dominguez R, Lopez M, Risueno N. Pseudo-angiomatous stromal hyperplasia presenting as a breast mass: imaging findings in three patients. Breast. 2004;13(5):431-5.
- Leon ME, Leon MA, Ahuja J, Garcia FU. Nodular myofibroblastic stromal hyperplasia of the mammary gland as an accurate name for pseudoangiomatous stromal hyperplasia of the mammary gland. Breast J. 2002;8(5):290-3.
- Levine PH, Nimeh D, Guth AA, Cangiarella JF. Aspiration biopsy of nodular pseudoangiomatous stromal hyperplasia of the breast: clinicopathologic correlates in 10 cases. Diagn Cytopathol. 2005;32(6):345-50.