Case Report
Pseudocirrhosis in Patients with Metastatic Breast Cancer after Treatment with Eribulin
Nadiye Akdeniz, Muhammet Ali Kaplan*, Mehmet Küçüköner, Zuhat Urakçı, Oğur Karhan and
Abdurrahman Işıkdoğan
Department of Medical Oncology, Dicle University, Diyarbakır, Turkey
*Corresponding author: Muhammet Ali Kaplan, Department of Medical Oncology, Dicle University, Sur/ Diyarbakir, Turkey
Published: 17 Sep, 2018
Cite this article as: Akdeniz N, Ali Kaplan M, Küçüköner
M, Urakçı Z, Karhan O, Işıkdoğan
A. Pseudocirrhosis in Patients with
Metastatic Breast Cancer after
Treatment with Eribulin. World J Surg
Surgical Res. 2018; 1: 1057.
Abstract
Pseudocirrhosis, an important complication of metastatic disease, is rarely seen and most commonly
described in patients with breast cancer. Despite its radiological and clinical similarity with cirrhosis,
it is different pathophysiologically. Eribulin, a novel synthetic chemotherapeutic agent, is one of the
few choices of treatments that prolong overall survival in metastatic breast cancer who previously
treated with multiple chemotherapy regimens. Herein we report two patients with breast cancer and
liver metastasis who developed pseudocirrhosis after achieving a clinical and radiographic response
to eribulin.
Keywords: Breast cancer; Pseudocirrhosis; Eribulin
Introduction
Breast cancer is the most common malignant disease diagnosed in women and affects one in
eight women over a lifetime in the world. At the time of diagnosis approximately 40% of women
presented with invasive breast cancer have regional spread or distant metastases and during the
course of the disease about half of metastatic breast cancer patients have metastasis to the liver.
Treatment of these patients presents a difficult clinical problem with the involvement of the liver
[1]. Because of several commonly used cytotoxic drugs in the treatment of advanced breast cancer
are activated or metabolized by the liver, administration of chemotherapy can be complicated [2] .
Systemic chemotherapy has well known hepatotoxic effects which are increasement serum levels of
hepatic enzymes, fatty infiltration of the liver, focal hepatitis, portal fibrosis, pseudocirrhosis, and
hepatic necrosis [3]. Pseudocirrhosis radiographically like macronodular cirrhosis and can cause
hepatic decompensation, whereas histopathologically cirrhosis is absent. Liver metastatic breast
cancer treated with chemotherapy is the most prominent cause of pseudocirrhosis [4].
Eribulin, a novel synthetic chemotherapeutic agent, is microtubule inhibitor differs from
Taxanes by the site of action. Eribulin is one of the few choices of treatments that prolong overall
survival in metastatic breast cancer who previously treated with multiple chemotherapy regimens
[5]. We report two patients with breast cancer and liver metastasis who developed pseudocirrhosis
after achieving a clinical and radiographic response to eribulin.
Case Presentation
Case 1
A 56-year-old female patient was diagnosed with invasive ductal adenocarcinoma of the left
breast in the locally advanced stage. After surgery, adjuvant chemotherapy with FEC (5-Fluorouracil,
Epirubicin, Cyclophosphamide) and Docetaxel was administered followed by locoregional
radiotherapy and Letrozole. After 19 months, multiple metastatic hepatic lesions were detected.
The patient was treated by weekly Paclitaxel, Gemcitabine, Docetaxel and Capecitabine inorderly.
After this treatment progression was observed and eribulin was initiated. Before eribulin treatment
laboratory findings were: hemoglobin: 13.2 g/dL; leukocyte count: 4.850 mm³; platelet count:
298.400 mm³; albumin: 3.4 g/dL; total protein: 6.95 g/dL; Aspartate Aminotransferase (AST): 165
U/L; Aminotransferase (ALT): 99 U/L; Alkaline Phosphatase (ALP): 142 U/L; Gamma-Glutamyl
Transpeptidase (GGT): 846 U/L; Lactate Dehydrogenase (LDH): 278U/L; total bilirubin: 1.72 mg/
dL. Hepatitis B and C viral infection markers were negative. As tumor markers Carcinoembryonic
Antigen (CEA) was 5.4 ng/mL and serum Carbohydrate Associated Antigen (CA) 15-3 was 616 U/
mL (normal range < 25 U/mL). F18-Fluorodeoxyglucose Positron emission tomography (18F-FDG
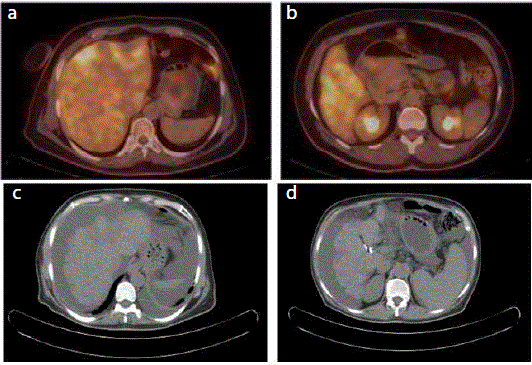
PET-CT) showed bone metastases and bilobar liver lesions with SUVmax value (standardized uptake value) 5,7 which has hypometabolic center because of necrosis (Figure 1A and 1B). A follow-up 18F-FDG PET CT performed after 6 cycles
of eribulin showed the development of decreased hepatic volume,
irregular contours, heterogeneously increased activity, regression
in metastatic lesions and newly developed ascites (Figure 1C and
1D). After eribulin treatment laboratory findings were: hemoglobin:
10.2 g/dL; leukocyte count: 3.540 mm³; platelet count: 92.860 mm³;
albumin: 2.6 g/dL; total protein: 6.9 g/dL; AST: 29 U/L; ALT: 13 U/L;
ALP: 72 U/L; GGT: 78 U/L; LDH: 206 U/L; total bilirubin: 0.7 mg/dL;
International Normalized Ratio (INR): 1.09. CA15-3 had decreased
to 52.95 U/mL. Serum-ascites albumin gradient was calculated as
1.7. Ascites cytology was evaluated as benign. She could not continue
treatment with eribulin later due to toxicity. The other agents were
inappropriate because of performance status. The patient died after 24
months of liver metastasis due to hepatic failure caused by metastasis.
Case 2
A 47-year-old female patient was consulted for left breast mass
which was diagnosed as invasive ductal adenocarcinoma by biopsy.
Liver metastasis was detected with imaging. Doxorubicin with
Cyclophosphamide subsequently Docetaxel with Trastuzumab
treatments were given to the patient for palliative treatment.
Follow-up Computerized Tomography (CT) scan performed after
chemotherapy showed complete regression of the liver metastasis.
Then she underwent mastectomy and radiation therapy was applied
followingly. Trastuzumab treatment completed to one year. During
follow-up, recurrence with multiple liver and newly bone metastases
was detected. The patient was treated in-orderly by Lapatinib
with Capecitabine, Navelbin with Trastuzumab, Paclitaxel with
Carboplatin and one agent Gemcitabine for palliation. After this
treatment progression was observed in the liver and bone a lesion by
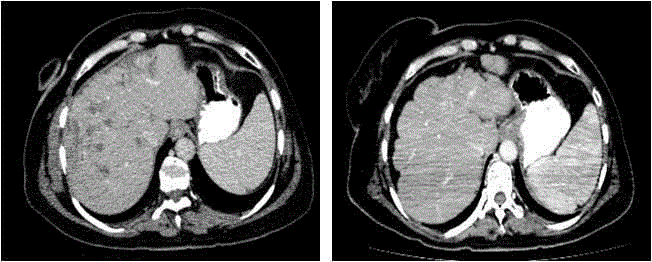
CT scan and eribulin therapy was started (Figure 2A). Before eribulin
treatment laboratory findings were: hemoglobin: 9 g/dL; leukocyte
count: 15.370 mm³; platelet count: 123.000 mm³; albumin: 2.7 g/dL;
total protein: 6.4 g/dL; AST: 54 U/L; ALT: 40 U/L; ALP: 388 U/L;
GGT: 722 U/L; LDH: 253 U/L; total bilirubin: 1.49 mg/dL. Hepatitis
B and C viral infection markers were negative. As tumor markers
Carcinoembryonic Antigen (CEA) was 16 ng/mL (normal range
< 5 ng/mL) and serum Carbohydrate Associated Antigen (CA) 15-3
was 48 U/mL (normal range < 25 U/mL). CT scan performed after 8
months of eribulin treatment showed regression of liver metastases,
liver parenchyma heterogeneity, lobular appearance, capsule
retraction and splenomegaly (Figure 2B). After eribulin treatment
laboratory findings were: hemoglobin: 12 g/dL; leukocyte count:
4.220 mm³; platelet count: 72.890 mm³; albumin: 2.4 g/dL; total
protein: 5 g/dL; AST: 37 U/L; ALT: 30 U/L; ALP: 245 U/L; GGT: 390
U/L; LDH: 252 U/L; total bilirubin: 0.57 mg/dL; INR: 0.93. CA15-3
had decreased to 26 U/mL and CEA had decreased to 0.78 ng/mL. On
the 22nd month of treatment with eribulin progression was observed
and treatment was continued with Trastuzumab emtansin. After 3
cycles patient died of hepatic failure caused by metastasis.
Figure 1
Figure 1
F18 FDG PET CT axial and fusion images (A,B) show increased
activity in bilober hepatic metastases before eribulin treatment. After 6 cycles
of eribulin CT scan (C,D) shows regression in metastatic lesions ,decreased
hepatic volume, irregular contours consistent with pseudocirrhosis and newly
developed ascites as the sign of portal hypertension.
Figure 2
Figure 2
Contrast-enhanced CT axial image (A) before eribulin treatment
shows multiple liver metastases. After 8 months of eribulin CT scan (B)
shows regression in metastatic lesions, liver parenchyma heterogeneity,
lobular appearance, capsule retraction consistent with pseudocirrhosis and
splenomegaly as the sign of portal hypertension.
Discussion
Pseudocirrhosis, an important complication of metastatic disease,
is rarely seen and most commonly described in patients with breast
cancer [6]. Despite its radiological and clinical similarity with cirrhosis,
it is different from cirrhosis pathophysiologically. It has got the same
morphological changes as cirrhosis including parenchyma atrophy,
nodularity, capsular retraction, and caudate lobe hypertrophy [7,8].
Chemotherapy administered liver metastatic breast cancer is the most
commonly reported cause of pseudocirrhosis, but it can be associated
with other metastatic diseases, including pancreatic cancer, colon
cancer, medullary thyroid cancer and esophageal cancer [4,9-11].
Pseudocirrhosis occurred by multifactorial mechanism
including; scarring and capsular retraction caused by chemotherapy
as a result of treatment response, hepatic metastasis encircled by
fibrous tissue, and chemotherapy-induced hepatic ischemia result in
nodular regenerative hyperplasia. Another proposed mechanism is
chemotherapy-induced sinusoidal obstruction [8,12,13].
While some patients diagnosed incidentally in an asymptomatic
period on surveillance imaging, typically many patients present with
portal hypertension complications such as ascites, portosystemic
venous collaterals, and splenomegaly [13].
The exact prevalence of pseudocirrhosis is not known. In one
retrospective study, all 22 liver metastatic breast cancer patients had
pseudocirrhosis detected by abdominal CT scans whereas 52% and
27% of cases had ascites and splenomegaly, respectively [14].
Qayyum et al. reviewed data of 91 patients treated with
chemotherapy for breast cancer with hepatic metastasis
retrospectively. Hepatic contour abnormalities were detected in 75%
of patients during 15 months median follow-up period. Sixteen of
68 patients developed diffuse nodularity resembling cirrhosis. Portal
hypertension developed in 6 of 16 patients with diffuse nodularity
and 1 of 42 patients with limited contraction [8]. In another study,
29(50%) of 58 patients with hepatic metastasis from breast cancer had
various degrees of hepatic capsular retractions [15].
In our cases, CT scan showed capsule retraction and lobular
appearance consisted with pseudocirrhosis related to treatment
response which was disappearance of the liver lesion by CT
and decrease level of tumor markers. In both cases, ascites and
splenomegaly occurred as a complication of portal hypertension.
A wide range of chemotherapeutic agents like Adriamycin,
Cyclophosphamide, 5-Fluorouracil, Methotrexate, Cisplatin,
Gemcitabine, Tamoxifen, Paclitaxel, Capecitabine, Trastuzumab and
Navelbine may cause pseudocirrhosis in patients with breast cancer
[8,14,16].
To our knowledge, this represents the first reported case of
pseudocirrhosis arising in the setting of regression of liver metastases
from breast cancer treated with eribulin.
In conclusion, pseudocirrhosis can be diagnosed in tumor
metastasis, progression, and chemotherapy responsive patients.
Histopathologic evaluation of liver biopsy is crucial but it is invasive,
on the other hand, most cases can be diagnosed by tumor markers
and imaging methods like in our cases. It must be remembered that
monitorization of hepatic decompensation and portal hypertension
complications are as important as the cancer treatment.
References
- Diamond JR, Finlayson CA, Borges VF. Hepatic complications of breast cancer. Lancet Oncol. 2009;10(6):615-21.
- Benjamin RS, Wiernik PH, Bachur NR. Adriamycin chemotherapy-efficacy, safety, and pharmacologic basis of an intermittent single high-dosage schedule. Cancer. 1974;33(1):19-27.
- Ngo D, Jia JB, Green CS, Gulati AT, Lall C. Cancer therapy related complications in the liver, pancreas and biliary system: an imaging perspective. Insights Imaging. 2015;6(6):665-77.
- Kang SP, Taddei T, McLennan B, Lacy J. Pseudocirrhosis in a pancreatic cancer patient with liver metastases: a case report of complete resolution of pseudocirrhosis with an early recognition and management. World J Gastroenterol. 2008;14(10):1622-4.
- Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet. 2011;377(9769):914-23.
- Jüngst C, Krämer J, Schneider G, Lammert F, Zimmer V. Subacute liver failure by pseudocirrhotic metastatic breast cancer infiltration. Ann Hepatol. 2013;12(5):834-6.
- Jeong WK, Choi S-Y, Kim J. Pseudocirrhosis as a complication after chemotherapy for hepatic metastasis from breast cancer. Clin Mol Hepatol. 2013;19(2):190-4.
- Qayyum A, Lee GK, Yeh BM, Allen JN, Venook AP, Coakley FV. Frequency of hepatic contour abnormalities and signs of portal hypertension at CT in patients receiving chemotherapy for breast cancer metastatic to the liver. Clin Imaging. 2007;31(1):6-10.
- Kobashigawa C, Nakamoto M, Hokama A, Hirata T, Kinjo F, Fujita J. Pseudocirrhosis in metastatic esophageal cancer. South Med J. 2010;103(5):488-9.
- Harry BL, Smith ML, Burton JR Jr, Dasari A, Eckhardt SG, Diamond JR. Medullary thyroid cancer and pseudocirrhosis: case report and literature review. Curr Oncol. 2012;19(1):e36-41.
- Battisti S, Guida FM, Pagliara E, Tonini G, Zobel BB, Santini D. Pseudocirrhosis after anti-EGFR-based neoadjuvant therapy for hepatic metastasis from colon cancer: A different point of view. Clin Colorectal Cancer. 2014;13(3):e13-5.
- Sass DA, Clark K, Grzybicki D, Rabinovitz M, Shaw-Stiffel TA. Diffuse desmoplastic metastatic breast cancer simulating cirrhosis with severe portal hypertension: A case of ‘pseudocirrhosis’. Dig Dis Sci. 2007;52:749-52.
- Lee SL, Chang ED, Na SJ, Kim JS, An HJ, Ko YH, et al. Pseudocirrhosis of breast cancer metastases to the liver treated by chemotherapy. Cancer Res Treat. 2014;46(1):98-103.
- Young ST, Paulson EK, Washington K, Gulliver DJ, Vredenburgh JJ, Baker ME. CT of the liver in patients with metastatic breast carcinoma treated by chemotherapy: Findings simulating cirrhosis. AJR Am J Roentgenol. 1994;163(6):1385-8.
- Fennessy FM, Mortele KJ, Kluckert T, Gogate A, Ondategui-Parra S, Ros P, et al. Hepatic capsular retraction in metastatic carcinoma of the breast occurring with increase or decrease in size of subjacent metastasis. AJR Am J Roentgenol. 2004;182(3):651-5.
- Schreiner SA, Gorman B, Stephens DH. Chemotherapy related hepatotoxicity causing imaging findings resembling cirrhosis. Mayo Clin Proc. 1998;73(8):780-3.