Research Article
Discrepancy in Colorectal Cancer Staging: A Single Center Experience
Elfaedy O*, Owens P, Aakif M and Mansour E
Department of Surgery, St. Luke's General Hospital, Ireland
*Corresponding author: Elfaedy O, Department of Surgery, St. Luke's General Hospital, Ireland
Published: 17 Sep, 2018
Cite this article as: Elfaedy O, Owens P, Aakif M, Mansour
E. Discrepancy in Colorectal Cancer
Staging: A Single Center Experience.
World J Surg Surgical Res. 2018; 1:
1054.
Abstract
Background: The TNM (Tumor Node Metastases) staging system developed by the American Joint
Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) is the most
widely used classification system for colorectal cancer staging. Accurate predictions of the definitive
pathological disease stage using pre-operative clinical-radiological staging techniques are crucial to
facilitate timely oncological and surgical planning following diagnosis.
Aims: This study describes a cohort of patients treated for colorectal cancer in a general hospital. We
aimed to assess the accuracy of pre-operative clinical-radiological staging in predicting pathological
colorectal cancer stage at a non-specialist center.
Methods: A retrospective cohort study examined the records of 98 patients with histological
confirmed colorectal carcinoma over a 6 year period from 2010 to 2015. Ninety eight patients
were treated in St Luke’s General Hospital, while 14 patients were managed and followed up in
other hospitals. Data was collected by chart review and from prospectively maintained electronic
histopathology and radiology databases.
Results: Ninety eight cases of colorectal cancer were identified. The mean age at presentation
was 67.9 years; 50% patients were men; 26.5% had rectal tumors; and 85.7% underwent surgery
following clinical staging. Clinical radiological stage and pathological stage differed in 27.4% (n=23)
patients (p< 0.0001). Of those 23 patients, eight were up staged (none of whom received neoadjuvant
therapy), and 15 were down staged post operatively.
Conclusion: Discrepancy in staging colorectal cancer has critical effects on management, outcomes,
and survival rates of patients. Appropriate and accurate clinical radiological staging enables multi
disciplinary teams to plan optimal management approaches.
Keywords: Colorectal cancer; Cancer staging; Clinical radiological
Introduction
The TNM (Tumor Nodes Metastases) staging system of the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) is the most widely used classification system for staging malignant tumors [1]. The importance of malignant tumor staging has increased significantly over the past 30 years owing to a trend towards individualized therapies often dependent on accurate knowledge of disease progression. Inaccurate staging may result in suboptimal management; under staging of disease may give rise to inadequate treatment approaches, whereas over staging can result in morbidity associated with excess treatment [2]. This is especially true for neoplasm’s f the colon and rectum where there is a strong correlation between disease stage and prognosis [3,4]. This correlation has been established since the first colorectal cancer staging was developed by Cuthbert Dukes in 1929, when he classified rectal cancer based on its invasion into the bowel wall, through the bowel wall, or involving regional lymph nodes (Dukes A, B, and C, respectively) [5]. The AJCC TNM staging system provides a systematic method of colorectal cancer staging to aid with therapeutics planning. In the modern era of cancer management, neoadjuvant strategies are ubiquitous and close correlation between clinical-radiological and pathological staging is of great importance [6,7]. The goal of this study was the evaluation and comparison of clinicalradiological staging with pathological staging in a cohort of patients treated for colorectal cancer at a non-specialized general hospital. Clinical Radiological stage (rTNM) parameters are based on physical examination, imaging techniques, and biopsies of affected areas, whereas Pathological staging (pTNM) further incorporates intra operative findings and may only be elucidated following surgery to excise or explore the diseased colon [8].
Table 1
Table 2
Methods
This is a retrospective cohort study examined the records of
98 patients treated for colorectal cancer from 2010 to 2015 at St.
Luke’s Hospital, Kilkenny, patients are identified by interrogation
of the Hospital In-Patient Enquiry (HIPE) database, which classifies
disease based on the 10th revision of the International Statistical
Classification of Diseases (ICD-10) developed by the World Health
Organization (WHO) [9]. Patients with documented ICD-10 code
diagnoses from C18 to C20 (malignant neoplasm of the colon,
recto sigmoid and rectum) with histological confirmed colorectal
carcinoma over a 6 year period from 2010 to 2015 were included. A
data Performa sheet was used to record demographics and clinical
pathological parameters, which were gathered via chart review
and from prospectively maintained electronic histopathology and
radiological databases. Data recorded included age, sex, presenting
symptoms, mode of presentation, clinical radiological staging results
and surgical management strategies. Ethical approval was obtained
from the ethics committee in the hospital.
The AJCC cancer staging manual 7th edition (AJCC) guidelines
for TNM staging were used to determine staging parameters based on
clinical examination and imaging findings (Table 1 and 2) [10]. The
radiologist and pathologist recorded rTNM and pTNM designations,
respectively, and adhered to AJCC 7th edition TNM staging standards
for colorectal cancers. Computed Tomography (CT) of the thorax,
abdomen, and pelvis was routinely used to assess local and distant
tumor spread for all patients with colorectal cancer. In selected cases,
mostly for rectal cancers, Magnetic Resonance Imaging (MRI) of
the pelvis and/or abdomen in addition to ultrasound scanning and
colonoscopy examination was also utilized.
Pathological staging parameters were determined post operatively
by a colorectal specialist pathologist based in the University Hospital
Waterford (UHW), which is a designated cancer center under the
Irish National Cancer Control Program (NCCP). Tumor local
invasion, lymph node involvement, and resection margin status
were routinely reported by the pathologist. All pathology reports
specifically documented pTNM stage values. All cases were discussed
at multi disciplinary gastro-intestinal oncology team meetings.
Overall disease staging was compared with the pre operative
clinical radiological stage. Changes in individual pTNM parameters,
including local tumor size and extent (T), lymph node involvement
(N), and distant metastases (M) were individually examined. Statistical
analysis was performed using SPSS v20 and Pearson’s chi square test
was utilized for comparing frequencies of categorical variables.
Results
We identified 98 patients with colorectal cancer for evaluation.
The mean age at presentation was 67.9 years (+/- SD 12.1); 50%
(n=49/98) were men; 26.5% (n=26/98) had rectal tumors, and 73.5%
(n=72/98) had colon cancer. At presentation, 87% (n=85/98) of our
patients had T3/T4 tumors. Following clinical-radiological staging,
85.7% (n=84/98) received surgery. Of patients with rectal tumors,
96% (n=25/26) received surgery with curative intent, 17 of whom
received neoadjuvant therapy. Of patients with colon cancer, 81.9%
(n=59/72) received surgery, one of whom received neoadjuvant
treatment for a rectosigmoid lesion.
All patients had a contrast-enhanced Computed Tomography
(CT) scan of the thorax, abdomen, and pelvis. Additionally, baseline
serum Carcinoembryonic Antigen (CEA) levels were measured as
part of clinical staging. Colonoscopy was performed in all patients to
obtain tumor biopsies and to assess for synchronous colonic lesions.
Rigid sigmoidoscopy was performed in patients with rectal tumors.
MRI of the pelvis was performed in 61.5% (n=16/26) of patients
with cancers involving the rectum. Of the 10 patients who did not
undergo MRI, seven were diagnosed with rectosigmoid tumors,
and one did not proceed to surgery due to the extent of disease
burden and comorbid conditions. The remaining two patients had
contraindications to MRI.
Most patients (72.4%; n=71/98) were diagnosed following
presentation in an elective setting, following general practitioner or
emergency department referral to the surgical outpatient department,
referral from another hospital department, or during endoscopic
surveillance. The remaining 27.6% (n=27/98) were admitted through
the Emergency Department with acute presentations. The most
common presenting symptoms among colorectal cancer patients
were per rectal bleeding, unexplained weight loss, and abdominal
pain. As expected, more patients with rectal tumors presented with
rectal bleeding than with colon lesions (73.1% vs. 48.6%; Table 3).
Of 84 patients who received resectional surgery, 94.0% (n=79)
had an R0 complete resection reported on histopathology; 4.8%
(n=4/84) had an R1 resection (microscopic residual cancer), and 1.2%
(n=1/84) had an R2 resection (macroscopic residual cancer). The most
frequently performed operation was a sigmoid colectomy (26.2%,
n=22/84), followed by anterior resection and right hemicolectomy,
each at 23.8% (n=20). The next most frequent procedures were
extended right hemicolectomy (13.1%, n=11/84), abdominoperineal
resection 10.7%, n=9/84), subtotal colectomy (2.4%, n=2/84), and left
hemicolectomy (1.2%, n=1/84).
Overall disease staging
Of patients who underwent surgery, 27.4% (n=23/84)
demonstrated discordance between the clinical-radiological and the
pathological colorectal disease stage (p< 0.0001), as defined by the
AJCC cancer staging manual (7th edition) guidelines (Table 4). The
pathological stage differed from clinical-radiological staging in 22.7%
(n=15/66) of the cases where pre operative neoadjuvant treatment
was not given (p< 0.0001). Of the 23 patients with variations in postoperative
disease staging (Table 4), eight patients were up-staged
(none of whom received did not receive Neoadjuvant therapy),
whereas 15 patients were down staged (seven of none of whom
receive neoadjuvant therapy). In two cases, there were variations in
TNM staging parameters without a resulting change in the overall
disease stage.
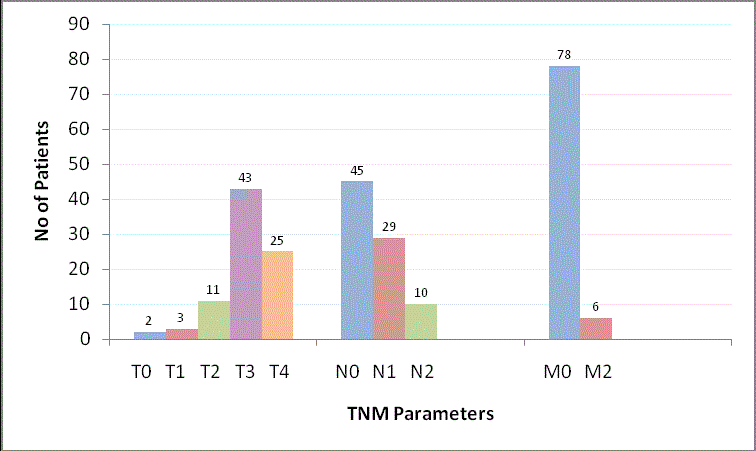
Primary tumor staging(T)
Variation in Tumor (T) staging was noted in 13.1% (n=11/84)
of patients at pathological staging compared to pre-operative
clinical-radiological staging (Table 5). Four of these patients had an
increase in T stage, none of whom had Neoadjuvant treatment. At
histopathology, primary tumor stage pT3 occurred most frequently
(in 51.2% of patients; n=43/84; Figure 1). Two patients had an
increase in stage from rT3 to pT4, one from rT2 to pT3, and the
remaining patient had a pT1 tumor that was not detected on imaging,
and pre-operatively staged as rT0 [11].
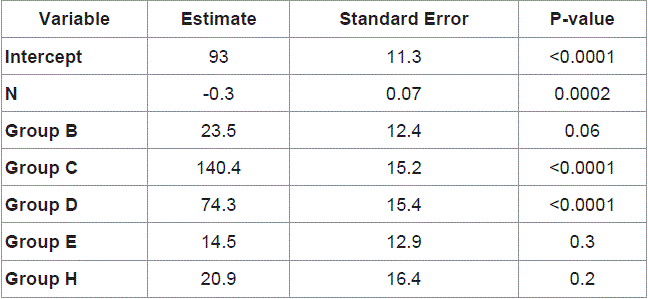
Regional lymph node staging (N)
The greatest discrepancy in individual TNM staging parameters
was found in nodal disease (N) staging, where 17.9% (n=15/84) of
cases differed between pathological staging and clinical radiological
staging (Table 5). Nodal disease burden was detected in 46.4%
(n=39/84) of pathology specimens, whereas node positivity was
radiographically evident in 54.8% (n=46/84).
Clinical radiological staging of lymph node metastases was
concordant more often in patients with rectal disease (87.5%,
n=7/8) than in those with colon tumors (84.7%, n=50/59). In one
patient with rectal disease, N stage increased from rN1 to pN2,
despite Neoadjuvant therapy. Out of 67 patients who did not receive
Neoadjuvant therapy, 15.2% (n=10) had a change in N-stage; in three,
the stage increased from rN0 to pN1 and in one, from rN1 to pN2.
Down-staging occurred in six patients: in four, the stage decreased
from rN1 to pN0; in one from rN2 to pN1; and in one from rN2 to
pN0.
Distant metastasis staging (M)
In patients with colorectal cancer, 22.6% (n=19/84) had
metastases identified radiologically; three were patients with rectal
disease and 16 had primary colonic disease. Fourteen of these patients
did not proceed to surgery. Of the five who proceeded to surgery,
two were patients with rectal tumors who both received Neoadjuvant therapy. One remained in Stage 4, while the second was down staged
to stage 3 following treatment. The remaining three patients had
colonic tumors, and proceeded to surgery with the stage in one being
restaged as pM0 following surgery. Metastatic disease was found
in six patients at surgery; in three, metastases were not detected by
preoperative investigations.
Figure 1
Table 3
Table 4
Table 4
Variation in post-operative pathological disease staging compared to clinical-radiological staging.
Table 5
Table 5
Post-operative pathological TNM variations, as compared to clinical-radiological TNM parameters.
Discussion
Although surgery remains the mainstay of colorectal cancer
management in the modern era, overall treatment strategies
increasingly include both adjuvant and Neoadjuvant radiotherapy
and chemotherapeutic modalities [5,12]. Treatment strategies
are formulated following multi disciplinary team discussion, as
in this study. Provision of personalized treatment regimens gives
rise to increasing permutations and combinations of management
approaches. Arriving at the optimal management plan for any given
patient necessitates a complex decision making process, which relies
heavily on clinical-radiological staging. Neoadjuvant therapy is now a
frequently utilized modality for many advanced gastrointestinal solid
tumors, including rectal, esophageal, and gastric cancers [13,14].
Pre operative chemotherapy has not yet been adequately proven
for colonic tumors, although it is being investigated because earlier
treatment may be more effective at eradicating micro metastatic
disease when compared to systemic treatment months after surgery.
Thus, recurrence rates may be reduced and rates of curative resection
potentially increased. Recently, the FOxTROT randomized control
trial group reported a study of the feasibility, safety, and efficacy of
neoadjuvant treatment for advanced colon cancers [14].
The profile of clinical radiological presentations in our cohort
compared to published presentations.
In this cohort, Pre-operative clinical-radiological evaluation
and diagnostics were inaccurate for nearly a quarter of all patients
who did not receive Neoadjuvant treatment for colorectal cancer.
Eight patients were up-staged and seven down staged following
pathological staging. Incorrect over-staging may have significant
impact on morbidity and mortality secondary to over-treatment.
Conversely, insufficient treatment of inaccurately under staged
patients may cause increased rates of metastatic and recurrent disease.
Best practice guidelines recommend CT of the thorax, abdomen,
and pelvis for staging of all colorectal tumors. MRI of the pelvis or
endoanal ultrasound is recommended for patients with rectal disease.
Full colonoscopy is also a minimum requirement, usually performed
pre operatively, but may be performed post-operatively in patients
who present with primary tumors not navigable endoscopically or in
emergency presentations [12,15].
Neoadjuvant chemo-radiotherapy is an increasingly utilized
strategy for management of patients with rectal tumors, especially for
those with locally advanced or node positive disease, and commonly
includes options such as short course pre operative radiotherapy or
chemo-radiotherapy [16,17]. MRI and endoanal ultrasound assist
in identifying appropriate candidates for Neoadjuvant management
options. These investigations evaluate both T and N stage, and examine
mesorectal nodal burden and mesorectal fascia involvement [16-18],
which is essential for surgical planning. MRI aids in measuring the
distance from the anal verge, thereby aiding the surgeon’s decision
on the optimal surgical strategy: Abdomino-perineal resection or
sphincter saving ultra-low anterior resection, which is permissible to
a distance of 1 cm from the anal verge in contemporary guidelines
[15]. The identification of nodal disease is important for decisions
regarding Neoadjuvant therapy in rectal disease. This has proven a
difficult diagnostic task for radiologists and is often based on lymph
node shape and size identified on MRI, with nodes >8 mm deemed
positive on imaging for clinical staging [15].
CT is the most frequently utilized modality for colorectal cancer
staging, with a reported accuracy ranging from 45% to 77% for
the assessment of nodal metastases [16,18,19]. MRI has a reported
staging accuracy of 73% with a sensitivity of 40% for lymph node
metastases detection. The addition of MRI to CT further improves
the overall accuracy of staging rectal cancer [16-18]. Access to clinical
and radiological staging resources played a role in the accuracy
of colorectal cancer staging in our cohort. CT is undertaken on
site at our institution, whereas MRI, although accessible via other
institutions, is not readily available on site. Lack of direct access to
PET/CT and endoanal ultrasound scanning may also have a negative
impact on optimal staging. Although PET/CT is not routinely
recommended for initial staging of colorectal cancer, it is frequently
used to clarify equivocal findings on traditional modalities such
as CT and MRI, especially in the setting of potential metastatic
disease and synchronous lesions where colonoscopy cannot be
completed due to obstructing tumors. For staging of rectal cancer,
MRI was typically used to augment CT in our cohort, with no use of
endoanal ultrasound. Although there is limited conclusive evidence
to demonstrate superiority of one modality over the other, endoanal
ultrasound is generally beneficial in rectal T staging, especially for
early disease, whereas MRI improves visualization of mesorectum
and circumferential resection margin involvement for more advanced
tumors [16-18]. Access to MRI and PET/CT modalities and the
availability of endoanal ultrasound may have improved the accuracy
of clinical radiological staging in our cohort.
Variability in staging may also alter the management approach
in colonic lesions. Pre operative CT can identify loco-regional tumor
extent, lymphatic and distant metastases, and disease complications
such as perforation or fistula formation. Differentiation between T3
and T4 tumors aids in planning the surgical approach, when tumor
extension into surrounding structures may necessitate more extensive
resection, possibly with surgical interdisciplinary cooperation required
at surgery. Especially for our center, T4 tumors typically necessitate
referral to centers where urologic and gynecologic oncology input
is available for multi disciplinary procedures. In addition, accurate
assessment for liver metastases, initially with CT and then MRI or
ultrasound scanning pre-operatively, allows for optimal surgical
planning for metastatic tumors [20]. PET/CT is valuable in selected
cases for localizing synchronous colorectal cancers proximal to
obstructing lesions, and for evaluating extra colonic and hepatic
disease. However, there is no robust evidence to support the routine
use of PET/CT for staging [20]. There are increasingly improved
outcomes for patients following curative resection of liver metastases.
Five year survival (58%) and overall recurrence rates (52%) have been
reported post liver resection [21]. Many surgeons opt for resection
of liver metastases in an index procedure followed by primary tumor
resection. The early identification of metastases facilitates a timely
transfer to the tertiary referral center for metastasectomy.
Colorectal cancer is a disease entity responsive to screening
given that it has a defined pathological progression and is highly
treatable if diagnosed early [13]. In cases of symptomatic patients,
timely diagnostics and prompt management result in improved outcomes [14]. Clarke et al. report an increase in late stage colorectal
disease presentations (Stage 3 to Stage 4) from 42% (n=1,752) to 50%
(n=2,298) following their interrogation of Ireland’s National Cancer
Registry for the period 1995 to 2010 [21]. In 2016, the Association
of Coloproctology of Great Britain and Ireland (ACPGBI) published
their national bowel cancer audit annual report, which examines
demographics and clinical pathological parameters of over 30,000
patients in England and Wales with colorectal cancer diagnosed
between April 1, 2014 and March 31, 2015 [13]. This ACPGBI
audit indicated an incidence of locally advanced T3/T4 tumors of
57% (n=17,220/30,122) at clinical radiological staging, which is less
than the 87% (n=85/98) of our patients who had T3/T4 tumors at
presentation. Our cohort exhibited node positivity in 54.8% of
patients at clinical-radiological staging, which is more frequent
than the 41% (n=12,420/30,122) reported by the ACPGBI [20]. The
metastatic disease incidence was 22.6% (n=19/84) in our cohort at
clinical radiological staging, whereas the ACPGBI reported 18.2%
(n=5,471/30,122). Thus, our rates of advanced disease at clinical
radiological staging appear higher than those reported in the national
bowel cancer audit. This increased rate of late presentations of
cancer at our hospital requires spending the least possible time from
presentation to clinical radiological workup.
Conclusion
High quality, accurate clinical-radiological staging enables multidisciplinary teams to plan optimal management strategies for patients with colorectal neoplasms. All patients presenting with red flag symptoms should be promptly investigated and staged according to international standards followed by discussion in a multi disciplinary setting prior to treatment. There is a disparity between clinicalradiological staging and pathological staging in our cohort, which may be improved with education for high risk patients and their families, priority access to endoscopy, and optimal radiological staging modalities such as CT, MRI, PET/CT, and endoanal ultrasound.
References
- Edge SB, Compton CC. The American joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471-4.
- Hermanek P, Sobin LH, Wittekind C. How to improve the present TNM staging system. Cancer. 1999;86(11):2189-91.
- Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54(6):295-308.
- Lindblom A. Improved tumor staging in colorectal cancer. New England J Med. 1998;339(4):264-5.
- Dukes CE. The classification of cancer of the rectum. J Pathol Bacteriol. 1932;35(3):323-32.
- Harisi R, Schaff Z, Flautner L, Winternitz T, Jaray B, Nemeth Z, et al. Evaluation and comparison of the clinical, surgical and pathological TNM staging of colorectal cancer. Hepatogastroenterology. 2008;55(81):66-72.
- Harisi R, Schaff Z, Winternitz T, Nemeth Z, Harsanyi L, Morvay K, et al. TNM staging at colorectal cancer. Zeitschrift für Gastroenterologie. 2005;43(05):40.
- American Joint Commitee on Cancer (AJCC). What is Cancer Staging? 2017[24 June 2017].
- Organization WH. International statistical classification of diseases and related health problems: World Health Organization; 2004.
- Turnbull RB, Jr KK, Watson FR, Spratt R. Cancer of the colon: The influence of the no-touch isolation technic on survival rates. Ann Surg. 1967;166(3):420-7.
- Moreno CC, Mittal PK, Sullivan PS, Rutherford R, Staley CA, Cardona K, et al. Colorectal cancer initial diagnosis: Screening colonoscopy, diagnostic colonoscopy, or emergent surgery, and tumor stage and size at initial presentation. Clin Colorectal Cancer. 2016;15(1):67-73.
- National Institute for Health and Care Excellence. Colorectal cancer: diagnosis and management. Clinical Guideline 131. London: NICE; 2014.
- The Association of Coloproctology of Great Britain and Ireland (ACPGBI) N, RCS. National Bowel Cancer Audit - Annual Report 2016. London: 2016.
- Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: The pilot phase of a randomised controlled trial. Lancet Oncol. 2012;13(11):1152-60.
- Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23(10):2479-516.
- McPhee SJ, Papadakis MA, Tierney LM. Current medical diagnosis and treatment 2010. New York: McGraw-Hill Medical; 2010.
- Dahnert W. Radiology Review Manual. Baltimore MD, USA: Lippincott Williams & Wilkins; 2003.
- Horton KM, Abrams RA, Fishman EK. Spiral CT of colon cancer: Imaging features and role in management. Radiographics. 2000;20(2):419-30.
- Choi AH, Nelson RA, Schoellhammer HF, Cho W, Ko M, Arrington A, et al. Accuracy of computed tomography in nodal staging of colon cancer patients. World J Gastrointest Surg. 2015;7(7):116-22.
- Kijima S, Sasaki T, Nagata K, Utano K, Lefor AT, Sugimoto H. Preoperative evaluation of colorectal cancer using CT colonography, MRI, and PET/CT. World J Gastroenterol. 2014;20(45):16964-75.
- Clarke N, McDevitt J, Kearney PM, Sharp L. Increasing late stage colorectal cancer and rectal cancer mortality demonstrates the need for screening: a population based study in Ireland, 1994-2010. BMC Gastroenterology. 2014;14(1):92.