Case Report
Primary Non-Hodgkin Lymphoma of the Maxilla
Alexoudi Vaia-Aikaterini1, Iskas Michail2, Anagnostopoulos Achilles2, Dimitriadis Ioannis3 and Antoniades Konstantinos*
1Department of Oral and Maxillofacial Surgery, Aristotle University of Thessaloniki, Greece
2Department of Hematology and BMT Unit, General Hospital of Thessaloniki, Greece
3Department of Pathology, General Hospital of Thessaloniki “G. Papanicolaou”, Thessaloniki, Greece
*Corresponding author: Antoniades Konstantinos, Department of Oral and Maxillofacial Surgery, Aristotle University of Thessaloniki, Greece
Published: 24 Aug, 2018
Cite this article as: Vaia-Aikaterini A, Michail I, Achilles
A, Ioannis D, Konstantinos A. Primary
Non-Hodgkin Lymphoma of the Maxilla.
World J Surg Surgical Res. 2018; 1:
1045.
Abstract
Lymphomas, or malignant lymphatic tissue neoplasms, can be classified as Hodgkin (HL) or Non- Hodgkin (NHL). Although head and neck lymphomas more frequently involve NHL, indicating extranodal proliferation, these cancers are relatively rare in the oral cavity. We report a case of a 56-year-old Caucasian woman who visited a dentist because of gradual buccal-side swelling near the upper left canine. The initial diagnosis was periapical abscess. She was later admitted to the department of Oral and Maxillofacial Surgery, Thessaloniki, Greece because of persistent swelling, where a diagnosis of primary NHL of the oral cavity was established following radiographic, histological and immunochemical studies. The patient was referred to the Department of Hematology, where she received R-CHOP therapy, concurrent intrathecal chemotherapy and subsequent involved field radiation therapy. After treatment completion, the findings of scheduled PET-CT restaging were consistent with a complete metabolic response. The patient continues to undergo regular follow-up CT scans and physical examinations. Our case experience suggests that each aggressive oral lesion should be screened to detect possible malignancy. An excisional biopsy and extensive histopathology evaluation should be performed prior to treatment initiation, and cooperation between hematologists and oral and maxillofacial surgeons is essential to early detection, diagnosis, and treatment and follow-up.
Introduction
Lymphomas, or malignant neoplasms of lymphatic tissue, are characterized by lymphocytic
proliferation. These cancers can be divided into two main types: Hodgkin (HL) and Non-Hodgkin
(NHL). The former type arises primarily within the lymph nodes, with extranodal involvement in
only 5% of cases. By contrast, the latter type exhibits extranodal involvement in approximately 24% to
40% of cases [1,2]. Extranodally, NHL most frequently involves Waldeyer’s ring, the gastrointestinal
tract, skin, bones, retroperitoneum, lung and central nervous system; by contrast, NHL of the oral
cavity is relatively rare [2].
Reported locations of NHL in the oral cavity include the tonsils, soft tissue, gingiva, bone, palate,
paranasal sinuses, floor of the mouth and salivary glands [2,3]. The oral manifestation of NHL is
usually secondary due to the dissemination of the disease. Primary oral manifestations are relatively
rare, with only a few recorded cases. NHL may be associated with swelling, pain, paresthesia of
the lip or osteonecrosis [4]. However, these nonspecific manifestations of NHL may mimic other
pathologic lesions, such as gingival swelling, periodontal disease, pericoronitis, apical radiolucency
or dental abscesses, and a definitive diagnosis may therefore be challenging [1,2,4,5].
Patients who are infected with the human immunodeficiency [6], have undergone solid organ and
hematopoietic stem-cell transplantation, have primary immunodeficiency syndromes, are receiving
immunosuppressive therapy and have experienced increased exposure to ultraviolet radiation are
reported to have a higher risk of developing NHL [7]. Moreover, various NHL subtypes have been
attributed to infection with microbial or viral pathogens such as the Epstein–Barr virus (Burkitt’s
lymphoma, extranodal NK-cell or T-cell lymphoma nasal type), Helicobacter pylori, Chlamydia
psittaci (mucosa-associated lymphoid tissue lymphoma) [8], human herpesvirus-8, human T-cell
lymphotropic virus-1, hepatitis C virus and simian virus 40. Currently, Diffuse Large B-Cell
Lymphoma (DLBCL), an aggressive lymphoid neoplasm with nodal or extranodal involvement,
is the most common subtype of NHL, accounting for approximately 80% of all lymphomas [2,4].
We report here a case of an extranodal DLBCL of the maxilla that presented as an odontogenic
radiolucent lesion, as well as the diagnosis and treatment of this rare involvement.
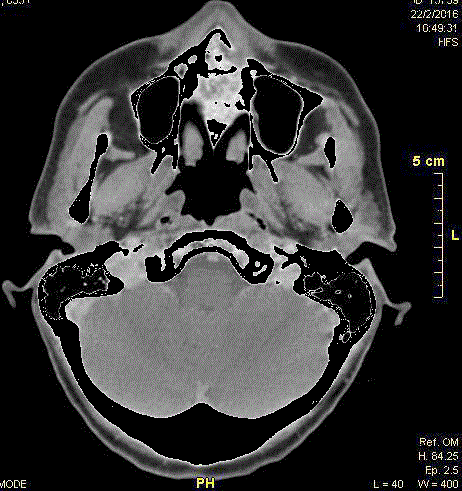
Figure 1
Figure 1
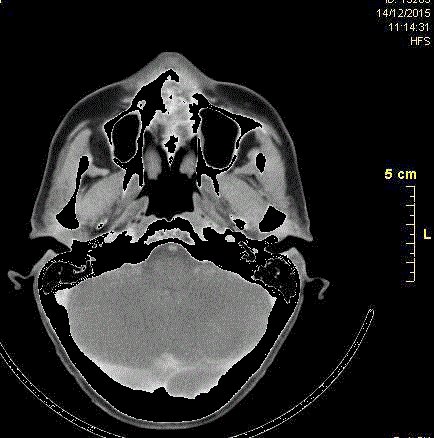
Axial computed tomography scan completed at the initial visit,
showing a large area of bony destruction within the anterior maxilla and
infiltrating the left sinus.
Figure 2
Figure 3
Case Presentation
A 56-year-old Caucasian woman presented with buccal-side
swelling near the upper left canine (tooth #23) that had evolved
gradually. She additionally complained of a sense of swelling in the
region beneath the nose and above the upper left canine. She had no
relevant medical history and no history of tobacco or alcohol use. The
patient had been referred to a dentist who administered antibiotics
and performed a root canal of the suspected tooth. The initial
diagnosis was a periapical abscess of the canine.
However, swelling persisted after the initial and shifted to the
palatal site of the suspected tooth. The patient was subsequently
admitted to the Department of Oral and Maxillofacial Surgery at our
institution, where an ordered Computed Tomography (CT) scan
revealed a large area of bone destruction (45 mm × 27 mm × 20 mm).
The lesion extended from the anterior medial and left aspect of the
maxilla relative to the roots of the left central and medial incisor and
canine and to the nasal cavity and inferior internal wall of the sinus
(Figure 1).
The maxillary lesion was biopsied and subjected to a pathologic
evaluation. Hematoxylin and Eosin (H&E) staining revealed a diffuse
infiltration of large lymphoid cells with irregular and gigantic nuclei,
multiple nucleoli and nuclear divisions (Figure 2). The neoplastic
cells exhibited the following staining characteristics: CD20+,
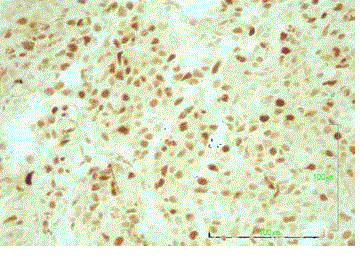
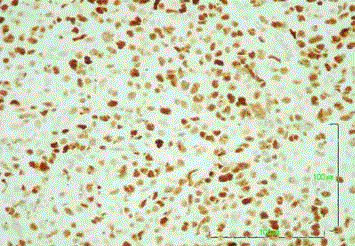
CD79a+, CD3-, CD5-, CD10+/-, MUM1+ and cyclin D1- (Figure 3
and 4). The cells were also positive for both Bcl-2 and Bcl-6; however,
c-myc and EBER staining was unavailable (Figure 5 and 6). Among
the neoplastic cells, the positive Ki67 rate was 55%, and a significant
reactive T cell population was detected (Figure 7). These morphologic
and immunohistochemical findings led to a diagnosis of DLBCL-Not
Otherwise Specified (NOS). The patient was subsequently referred to
the Department of Hematology.
The patient's serum immunoglobulin (Ig)G, IgA and IgM;
beta 2 microglobulin and lactic dehydrogenase levels were within
normal ranges, and her International Prognostic Index was 0 (low).
Additionally, she tested negative for HIV-1 and -2 and EBV. Disease
staging included CT scans of the head and neck, lung, abdomen and
pelvis; lumbar puncture for Cerebrospinal Fluid (CSF) evaluation and
bone marrow aspiration with trephine biopsy. Neither the imaging
scans nor bone marrow assessment indicated disease involvement.
The CSF examination also indicated no Central Nervous System
(CNS) involvement. The disease was classified as stage IE according
to the Ann Arbor staging system with extranodal involvement (E,
bone involvement of the maxilla).
The patient initially received three 21-day cycles of the
R-CHOP-21 regimen [rituximab, 375 mg/m2 intravenously (iv) on
day 1; cyclophosphamide, 750 mg/m2 iv on day 1; doxorubicin, 50
mg/m2; vincristine, 1.4 mg/m2 iv on day 1 and methylprednisolone,
100 mg orally on days 1-5]. Primary CNS prophylaxis comprising
intrathecal methotrexate 15 mg, cytarabine 50 mg and hydrocortisone
100 mg were administered on day 1 of each cycle. The patient
developed alopecia and grade 4 neutropenia after the first cycle, and
pegfilgrastim 6 μg was administered subcutaneously as a secondary
prophylaxis in each subsequent cycle to maintain grade 2 neutropenia.
The initial restaging CT scan showed a partial response of 60%
in the SPD of the lesion, and three more R-CHOP cycles were
administered without a further mass decrease. As per local protocol,
a PET-CT scan was performed and yielded findings consistent with a
complete metabolic remission. Accordingly, two additional R-CHOP
cycles were administered to complete a 6-month treatment regimen
as per local protocol, and involved site radiation therapy (total dose:
3,600 cGy) was administered to consolidate extranodal disease.
Repeated CT scans every 4 months revealed a pattern of reduction of
the lytic lesion (Figures 8-10), and a second biopsy of the affected area
(buccal site, #23) showed no signs of NHL recurrence.
The patient remains in complete remission at 27 months after
the initial diagnosis and 15 months after treatment completion. Her
performance status is excellent, and she has not exhibited evidence
of late toxicity.
Figure 4
Figure 5
Figure 5
Axial computed tomography scan after 3 cycles of chemotherapy,
showing a reduction of the lytic lesion.
Figure 6
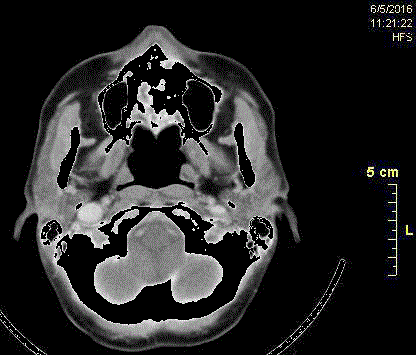
Figure 6
Axial recall CT scans showing reduction of the lytic lesion after 8
cycles of chemotherapy.
Figure 7
Figure 7
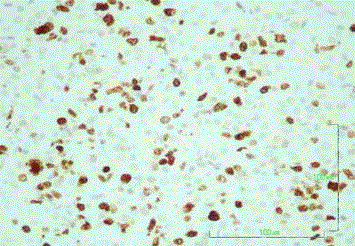
High-power view of the lesion biopsy reveals scattered large,
atypical lymphoid cells (hematoxylin and eosin (HE), magnification x400).
Figure 8
Figure 9
Figure 9
Axial recall CT scans showing reduction of the lytic lesion after 8
cycles of chemotherapy.
Figure 10
Discussion
Lymphoma is the second most frequent malignant lesion of the
head and neck region, after squamous cell carcinoma [2]. Although
the usual clinical manifestation is nodal disease, lymphoma may also
affect extranodal sites, as noted in the introduction. Waldeyer’s ring is
the extranodal site most commonly affected by NHL [9,10], whereas
the paranasal sinuses are rarely targeted (0.2% to 5% of cases) [11].
Regarding oral sites, the maxillary sinus is most commonly affected,
although lymphomas have also been reported in the soft tissues,
gingiva, bone, palate, floor of the mouth and salivary glands [3,4]. The
differential diagnosis includes similarly appearing conditions such as
dental abscesses or osteonecrosis [4], as well as infectious processes
such as systemic or deep mycosis and Wegener's granuloma, as
well as squamous cell carcinoma, metastatic tumors or neoplastic
process; notably, very rapid growth is a feature of both sarcomas and
lymphoproliferative disorders [12].
To our knowledge, fewer than 20 cases of NHL with a primary oral
manifestation have been reported worldwide, thus underscoring the
rarity of this disease. NHL of the jaws accounts for <5% and in case of
the mandible is 0, 6% of NHL of the bones. Paresthesia of the inferior
alveolar nerve may be a common finding of NHL of the mandible,
whereas pain and swelling are the most common manifestations of
NHL of the maxilla [13]. Overall, the most frequently reported subtype
of NHL is DLBCL, and the reported incidence of this subtype is even
higher among cases affecting the oral cavity (58% to 68%) [14,15]. The
median ages at diagnosis range between 67 and 71 years, although a
few cases have been described in younger individuals [1,14]. These
reports are consistent with the characteristics of our case. Given the
importance of early identification to the timely initiation of treatment
and a favorable outcome, these findings suggest that dental health
providers must have a high index of suspicion regarding this rare
clinical manifestation of NHL.
Currently, CT is considered to be the most useful preoperative
study for defining the initial dimensions of the mass. Radiographically,
NHL most commonly appears on CT images as an area of poorly
defined radiolucency (lytic pattern), as seen in our case, whereas
only a small percentage (~2%) of reported cases have exhibited
a radio-opaque (sclerotic) appearance. These radiolucent lesions
may resemble common odontogenic pathologies such as chronic
apical periodontitis and chronic periodontitis [9]. However, typical
generalized sclerosis of the affected bone has also been described
[16]. As these radiologic findings are not specific to lymphoma,
the clinicians consider that almost 80% of bone lesions exhibit an
osteolytic pattern, whereas the remaining 20% exhibit a sclerotic
pattern [2]. Accordingly, a diagnosis of NHL cannot be established
from radiographic criteria alone, and a histopathological evaluation
is required to establish early oncologic treatment [16]. Malignancy
should be considered for oral lesions with aggressive growth patterns
and poor responses to antibiotics and dental treatment, and excisional
biopsies should be performed for suspected lymphomas because a
differential diagnosis cannot be safely established via aspiration or
punch biopsy [17].
Currently, chemotherapy followed by radiation therapy is the
treatment of choice for DLBCL-NOS, and R-CHOP-21 is the most
commonly administered regimen. Histologic subtypes predictive
of inferior outcomes should be identified through extensive
immunohistochemistry and/or FISH and molecular studies; as such
cases require the use of more intensified protocols as R-CHOP or
Burkitt-like regimens [15]. Additionally, as patients with paranasal
sinus involvement have a high risk of CNS involvement, a lumbar
puncture and CSF examination should be included in the initial
staging. For cases with negative CSF findings, CNS prophylaxis
comprising intrathecal treatment or the addition of systemic
methotrexate for 6 cycles to 8 cycles is indicated. Reports of such
cases have described systematic consolidation with complementary
craniofacial radiotherapy, and this is always included in treatment
regimens [11]. Early diagnosis is required, and continuous monitoring
and close follow-up are strongly recommended for the early detection
of relapse.
References
- Kemp S, Gallagher G, Kabani S, Noonan V, O'Hara C. Oral non-Hodgkin's lymphoma: review of the literature and World Health Organization classification with reference to 40 cases. Oral Surg Oral Med Oral Pathol Endod. 2008;105(2):194-201.
- Angiero F, Stefani M, Crippa R. Primary non-Hodgkin's lymphoma of the mandibular gingiva with maxillary gingival recurrence. Oral Oncology Extra. 2006;42(3):123-8.
- Ratech H, Burke JS, Blayney DW, Sheibani K, Rappaport H. A clinicopathologic study of malignant lymphomas of the nose, paranasal sinuses and hard palate, including cases of lethal midline granuloma. Cancer. 1989;64(12):2525-31.
- Walter C, Ziebart T, Sagheb K, Rahimi-Nedjat RK, Manz A, Hess G. Malignant lymphomas in the Head and Neck region - a Retrospective, Single Center Study over 41 years. Int J Med Sci. 2015;12(2):141-5.
- Buchanan A, Kalathingal S, Capes J, Kurago Z. Unusual presentation of extranodal diffuse large B-cell lymphoma in the head and neck: description of a case with emphasis on radiographic features and review of the literature. Dentomaxillofac Radiol. 2015;44(3):20140288.
- Nenasheva VV, Nikolaev AI, Martynenko AV, Kaplanskaya IB, Bodemer W, Hunsmann G, et al. Differential gene expression in HIV/SIV-associated and spontaneous lymphomas. Int J Med Sci. 2005;2(4):122-8.
- Smedby KE, Hjalgrim H, Melbye M, Torrang A, Rostgaard K, Munksgaard L, et al. Ultraviolet radiation exposure and risk of malignant lymphomas. J Natl Cancer Inst. 2005;97(3):199-209.
- Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. The Lancet. 2012;380(9844):848-57.
- Wong DS, Fuller LM, Butler JJ, Shullenberger CC. Extranodal nonHodgkin's lymphoma of the head and neck. Am J Roentgenol, Radium Therapy Nuclear Med. 1975;123(3):471-81.
- Eisenbud L, Sciubba J, Mir R, Sachs SA. Oral presentation in nonHodgkin's lymphoma: a review of thirty-one cases. Part I. Data analysis. Oral Surg Oral Med Oral Pathol. 1983;56(2):151-6.
- Adouly T, Adnane C, Oubahmane T, Rouadi S, Abada R, Roubal M, et al. Uncommon Presentation of Non-Hodgkin’s Lymphoma of Maxillary Sinus: A Case Report. Am J Medical Case Rep. 2015;3(12):410-2.
- Agrawal MG, Agrawal SM, Kambalimath DH. Non-Hodgkins lymphoma of maxilla: A rare entity. Natl J Maxillofac Surg. 2011;2(2):210-3.
- Dinakar J, Priya L, Reddy S. Primary non-Hodgkin's lymphoma of the mandible. J Oral Maxillofac Pathol. 2010;14(2):73-6.
- Solomides CC, Miller AS, Christman RA, Talwar J, Simpkins H. Lymphomas of the oral cavity: histology, immunologic type, and incidence of Epstein–Barr virus infection. Hum Pathol. 2002;33(2):153-7.
- Urquhart A, Berg R. Hodgkin's and Non-Hodgkin's lymphoma of the head and neck. Laryngoscope. 2001;111(9):1565-9.
- Keating GM. Rituximab: a review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma. Drugs. 2010;70(11):1445-76.
- Deng D, Wang Y, Liu W, Qian Y. Oral and maxillofacial non-Hodgkin lymphomas: Case report with review of literature. Medicine (Baltimore). 2017;96(35):e7890.