Review Article
Use of Superior Mesenteric Vein for Renal Transplant Venous Outflow in a Patient with Extensive Inferior Vena Cava Thrombosis; a Case Report
Nalaka Gunawansa*
Department of Endovascular & Transplant Surgeon, Sri Lanka
*Corresponding author: Nalaka Gunawansa, Department of Endovascular & Transplant Surgeon, National Institute of Nephrology Dialysis and Transplantation, Sri Lanka
Published: 03 Aug, 2018
Cite this article as: Gunawansa N. Use of Superior
Mesenteric Vein for Renal Transplant
Venous Outflow in a Patient with
Extensive Inferior Vena Cava
Thrombosis; a Case Report. World J
Surg Surgical Res. 2018; 1: 1032.
Abstract
In patients with end stage renal failure having native iliac vein and inferior vena caval thrombosis, renal transplantation becomes a significant challenge with limited options for technical success. Alternate vascular beds need to be explored as venous outflow channels for the prospective allograft. The portal-mesenteric system is often spared in systemic venous thrombotic disease, offering an alternate venous outflow route. This requires careful planning and meticulous surgical expertise to achieve technical and functional success. Although lacking large numbers and long-term follow up, available results are encouraging and offers hope to such patients. We present our experience with such a patient where transplantation was done using the superior mesenteric vein and remains well at 15 months follow up.
Introduction
The routine vascular anastomosis in renal transplantation is performed to the recipient iliac
vessels. In pediatric recipients (weight < 15 kg) and certain re-transplants, venous and arterial
anastomosis may be performed to the Inferior Vena Cava (IVC) and aorta respectively [1,2].
Therefore, when the Common Iliac Veins (CIV) and IVC are both affected by systemic thrombosis,
possible venous outflow channels for transplantation are limited and challenging. It may often result
in significant delays or even being denied access to transplantation due to technical feasibility as well
as potential thrombotic complications post-transplant.
Background
S.N was a 28-year-old female in End Stage Renal Failure (ESRF), with systemic lupus
erythematosus and recurrent lower limb deep vein thrombosis since adolescence. She was on longterm
anticoagulation (warfarin) since the age of 16 and on maintenance haemodialysis (right sided
subclavian catheter) for 14 months. Her mother (47 years), came forward for live donation.
Assessment
Pre-operative duplex imaging of the patient showed an occluded left CIV. The right CIV though
patent showed monophasic flow with evidence of prior thrombotic disease and recanalization.
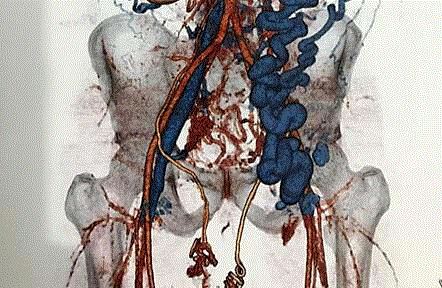
Further imaging with magnetic resonance angiography confirmed extensive thrombosis of left CIV
and infra-hepatic IVC (Figure 1). There was extensive collateral formation along the left inferior
epigastric, lumbar and retroperitoneal veins. The Superior Mesenteric Vein (SMV) and portal vein
were intact. Although the right CIV was patent, it appeared to be draining in to retroperitoneal
collaterals. The upstream thrombus and complete occlusion of the IVC and monophasic flow
within the right CIV were considered deterrents to successful graft implantation. The patent portalmesenteric
system was considered as a potential alternative. Detailed counseling was done for both
the donor and recipient regarding the possible outcomes and long-term benefits of transplantation
versus haemodialysis.
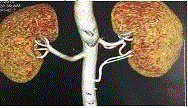
The donor renal angiogram showed two divergent arteries to left and a single artery to right
kidney (Figure 2). Differential renal functions were; Left 53%, right 47%. The right kidney was
selected for donation.
The recipient operation
Anticoagulation was changed from warfarin to enoxaparin, 4 days before surgery. The last dose
of enoxaparin was given on the eve of surgery, while keeping the patient on graduated compression
stockings throughout her hospital stay. A Midline laparotomy was
done, and preliminary vascular assessment was performed. The IVC
and aorta were exposed by medial rotation of the right colon; Cattell-
Braasch manoeuvre [3]. The IVC and left CIV were occluded with
palpable hard thrombi and severe inflammation in the peri-venous
tissue. The IVC thrombus extended beyond the confluence with
native renal veins. The portal and superior mesenteric veins were
patent and unaffected by thrombotic process. This was confirmed by
intra-operative on-table duplex imaging.
A right laparoscopic donor nephrectomy was performed in
conventional fashion. The retrieved kidney was cold perfused using
histidine-tryptophan-ketogluterate solution in the back-table. The
donor renal artery was short and was reconstructed with an extension
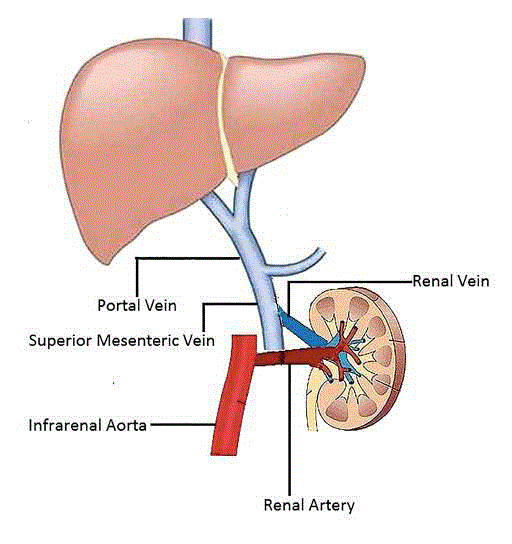
venous graft (recipient right reversed great saphenous vein). The
allograft vein was anastomosed to the proximal SMV (Figure 3); endto-
side configuration using 5/0 polypropylene. The reconstructed
renal artery was anastomosed end-to-side to the aorta also with 5/0
polypropylene. The total warm ischaemia time was 21 mins and cold
ischaemia time was 34 minutes. Immediate reperfusion was done and
showed excellent graft perfusion with minimal blood loss or systemic
effects on the recipient. The ureter was anastomosed to the native
right ureter (end-to-side), over a 5 French ureteric stent using 6/0
polydiaxone suture.
Peri-operative period
The immunesuppression was in keeping with that for a ‘lowimmunological
risk’ transplant, consisting of basiliximab induction,
tacrolimus, mycophenolate mofetil and prednisolone. Prophylactic
intravenous antibiotics were continued for 72 hrs after surgery
considering the extent of surgical dissection. The patient was
extubated immediately after surgery and was managed in the intensive
care isolation unit as for a standard transplant recipient. Oral feeding
was restricted to liquids in the first 24 hrs and solids were introduced
from day-02. Subcutaneous enoxaparin 20 mg daily was continued
from day-00.
The allograft showed immediate function with satisfactory
diuresis, achieving normal serum creatinine levels by day-03.
Warfarin was started at this time (day-03) and was continued along
with enoxaparin until therapeutic levels were achieved (day-07). At
this time, enoxaparin was discontinued, and warfarin was continued
at the same dose. Duplex imaging (day-01, day-04), showed excellent
graft perfusion and venous drainage. She was discharged on day-08
with normal graft function (serum creatinine 1.1 mg/dl).
Post-operative care
Initial post-operative visits showed sustained graft function
(serum creatinine 0.9-1.1 mg/dl). On day-27, she was admitted with
elevated serum creatinine, 1.9 mg/dl. Duplex scan showed high
arterial resistive indices (0.87-0.89) with normal venous drainage. The
blood tacrolimus level was 9.3 ng/ml. A biopsy was not performed
due to on-going anticoagulation and was treated empirically with
Methyl-Prednisolone Pulsing. Graft function returned to normal
with treatment and has been sustained since. Presently (15 months
post-operative), she maintains satisfactory graft function (Serum
creatinine 1.2 mg/dl) and remains in good health.
Figure 1
Figure 2
Figure 3
Discussion
In the absence of patent iliac veins for allograft venous drainage,
the alternatives are infra or supra-hepatic IVC, native renal veins
after native nephrectomy or mesentero-portal veins [4-6]. Successful
implantation to the portal vein, SMV and splenic vein in paediatric
recipients with deceased donor grafts has been reported with
reasonable success in small numbers [7-9].
We did not use the right CIV due to the extensive upstream
thrombosis in the IVC and monophasic flow on duplex. Extension of
the IVC thrombus beyond the confluence with renal veins precluded
implantation in to the native renal veins. The short length of the
right donor renal vein did not allow us to reach the portal vein, while
using the aorta for arterial anastomosis. The inferior mesenteric vein
although patent, appeared too delicate, thin walled and small. Hence
it was decided to use the larger SMV as the venous outflow. Postoperative
clinical and duplex surveillance did not show any impact on
graft function, native liver function or portal venous flow.
Conclusion
Renal transplantation offers the best outcomes for patients
in ESRF. Thrombosis of IVC and CIV should not be considered
contra-indications to renal transplantation thereby denying such
patients the chance for a transplant. While maintaining therapeutic
anticoagulation to prevent recurrent thrombosis, alternate venous
drainage routes such as the portal-mesenteric system should be
explored where feasible. Although deceased donor transplants
allow extra length of graft vessels to perform complex vascular
reconstruction, live donor transplants are limited by the short length
of graft artery and vein. Nevertheless, with meticulous planning
and care, live donors transplants can also be performed for the
selected individual patients. Available short-term results have shown
encouraging outcomes with excellent graft function.
Explicit informed written consent has been obtained from
the patient regarding the academic publication of this article with
relevant details.
Learning Points
• IVC thrombosis and thrombophilias are not a contraindication
for renal transplantation.
• The portal-mesenteric venous system is often spared in
systemic thrombotic disease.
• Successful transplantation can be done using the portalmesenteric
system with full anti-coagulation.
References
- Watson CJE, Friend PJ. Chapter 11–Surgical Techniques of Kidney Transplantation. In: Morris P, Knechtle SJ, editors. Kidney Transplantation–Principles and Practice. 2014:161-75.
- Adams J, Gudemann C, Tonshoff B, Mehls O, Wiesel M. Renal transplantation in small children--a comparison between surgical procedures. Eur Urol. 2001;40(5):552-6.
- Cattell RB, Braasch JW. A technique for the exposure of the third and fourth portions of the duodenum. Surg Gynecol Obstet. 1960;111:378-9.
- Pirenne J, Benedetti E, Kashtan CE, Llédo-Garcia E, Hakim N, Schroeder CH, et al. Kidney transplantation in the absence of the infrarenal vena cava. Transplantation. 1995;59(12):1739-42.
- Rosenthal JT, Loo RK. Portal venous drainage for cadaveric renal transplantation. J Urol. 1990;144(4):969-71.
- Aguirrezabalaga J, Novas S, Veiga F, Chantada V, Rey I, Gonzalez M, et al. Renal transplantation with venous drainage through the superior mesenteric vein in cases of thrombosis of the inferior vena cava. Transplantation. 2002;74(3):413-5.
- Patel P, Krishnamurthi V. Successful use of the inferior mesenteric vein for renal transplantation. Am J. Transplant. 2003;3(8):1040-2.
- Kumar S, Rathore Y, Guleria S, Bansal VK. Renal transplantation in a child with thrombosed inferior vena cava. Saudi J Kidney Dis Transpl. 2014;25(2):367-9.
- Millan M, Caicedo LA, Villegas JI, Serrano O, Caicedo L, Duque M, et al. Case report of cadaveric kidney transplantation with renal-portal venous drainage: A feasible way for a venous drainage in a complex generalized thrombosed vessels setting. Int J Surg Case Rep. 2016;28:192-5.