Review Article
Strangulation Obstruction and the Release of Strangulation. Effects of Fluid Administration on Mucosal Blood Flow and Damage
Kjell Ovrebo1*, Ellen Berget2, Ketil Grong3 and Jonas Fevang4
1Department of Surgery, Haukeland University Hospital, Norway
2Department of Pathology, Haukeland University Hospital, Norway
3Department of Clinical Science, University of Bergen, Norway
4Department of Orthopaedic Surgery, Haukeland University Hospital, Norway
*Corresponding author: Kjell K Ovrebo, Department of Surgery, Haukeland University Hospital, 5021 Bergen, Norway
Published: 13 Jul, 2018
Cite this article as: Ovrebo K, Berget E, Grong K, Fevang
J. Strangulation Obstruction and the
Release of Strangulation. Effects of
Fluid Administration on Mucosal Blood
Flow and Damage. World J Surg
Surgical Res. 2018; 1: 1031.
Abstract
Background/Purpose: This study evaluates bowel mucosa damage and the consequences of
crystalloid fluid administration on mucosa damages upon release from strangulation obstruction.
Secondary outcomes are metabolic and hemodynamic changes during and after release of
strangulation obstruction.
Methods: Twenty-four anesthetized pigs were subject to strangulation of the distal ileum for 185
min. Variables and specimens were registered and collected during strangulation and for 25 min
thereafter. Intravenous Ringer`s acetate infusion during strangulation obstruction and after release
of strangulation was 15 mL·kg-1·h-1 in group I (Standard infusion) and 55 mL·kg-1·h-1 in group II
(High infusion). Group III, (Sham) controls received 15 mL·kg-1·h-1 throughout the experiment.
Results: Strangulation obstruction reduced bowel blood flow from baseline averages of 2.9-3.8
ml·min-1·g-1 to 0.3-0.9 ml·min-1·g-1. Upon release of strangulation, the bowel blood flow remained
low in the standard infusion group but increased significantly towards baseline levels in the high
infusion group.
Strangulation damaged more mucosa with standard infusion (80% ± 13%) than high infusion (25%
± 6%) (p=0.032). Release of strangulation had no significant effect on the mucosa (72% ± 17% and
41% ± 15% damage, respectively). Mucosal cell proliferation fell during strangulation from 169
mm-1 ± 17 mm-1 in controls to 71 mm-1 ± 16 mm-1 in standard (p<0.05) and 120 mm-1 ± 16 mm-1
in high infusion group. Release of strangulation significantly increased cell proliferation towards
control levels.
Serum base excess decreased significantly during strangulation and release of strangulation in both
intervention groups. S-lactate increased significantly in blood from the strangulated loop, but only
in peripheral blood of the standard infusion group.
Conclusion: Careful observation for hypotension, tachycardia and biochemical changes related
to metabolic acidosis may contribute to early recognition of intestinal strangulation obstruction.
Enhanced intravenous fluid administration reduces bowel damages and hemodynamic consequences
of both strangulation and release of strangulation.
Reperfusion damages should not be expected upon release of strangulation in the strangulated
bowel and signs of bowel restitution appear early.
Keywords: Animal model; Experimental model; Intestinal microcirculation; Mucosa;
Reperfusion injury
Introduction
Strangulation obstruction occurs in 11% to 26% of cases with bowel obstruction [1,2].
Recognition of the diagnosis may be challenging and a delayed diagnosis is associated with nonviable
bowel, resection of wide areas of damaged bowel and increased risk of death [1]. Strangulation
involves concomitant partial occlusion of arterial inflow and venous drainage of the bowel in contrast
to complete mesenteric artery occlusion in ischemia [3]. The strangulation generates pronounced
bowel damage and the viability of the bowel is often difficult to
determine [4]. The decision to resect wide areas of damaged bowel is
often reached by the surgeon’s subjective judgment and may leave the
patient with a short bowel syndrome requiring long-term parenteral
nutrition [5]. Reluctance to preserve bowel during surgical salvage
procedures in strangulation obstruction is often justified by the
progression of bowel damage by reperfusion. Reperfusion damages
in the bowel are identified after release of arterial occlusion [6] and
several strategies have been tested in order to reduce the ischemic
reperfusion damages [7,8].
Whether release of strangulation obstruction also elicits
reperfusion damages in the bowel is unclear. In contrast, partial
restitution of bowel mucosa is observed 4 hr to 12 hr after the release
of strangulation [4,9]. Therefore, any reperfusion damage after release
of strangulation obstruction should be identified early after release of
strangulation.
Strangulation obstruction generates loss of extracellular fluids
and substitution with crystalloid fluids during strangulation
modulates mucosal blood flow and mucosal damage in pigs [10].
This study evaluates bowel mucosa damage and the consequences
of crystalloid fluid administration on mucosa damages upon release
from strangulation obstruction. Secondary outcomes are metabolic
and hemodynamic changes during and after release of strangulation
obstruction.
Materials and Methods
Animal preparation
Twenty-four locally bred domestic pigs weighing 32 kg ± 3 kg
(mean ± SD) were deprived of food overnight but had ad libitum
access to water. The animals received an intramuscular injection
of atropine 1 mg, diazepam 10 mg, and ketamine 300 mg prior to
mask induction of anaesthesia with isoflurane. All animals were
orotrachealy intubated and mechanically ventilated (Cato, Dräger,
Lübeck, Germany) to an end tidal CO2 concentration of 3.5 kPa
to 6 kPa. Inspiratory oxygen level (FiO2) was adjusted to keep
arterial saturation above 98%. Anaesthesia was maintained with
isoflurane (end tidal concentration below 1.7%) and a continuous
infusion of fentanyl 8 μg·kg-1·h-1 and midazolam 0.5 mg·kg-1·h-1, with
minor adjustments). Bolus injections of fentanyl/midazolam were
administered in case of reappearance of reflexes. Rectal temperature
was monitored and adjusted by means of a heating pad. A Venflon®
2 IV cannula (OD 1.0 mm) was inserted into a femoral artery for
measurement of arterial blood pressure, heart rate and for sampling
of peripheral arterial blood. Another catheter (OD 1.34 mm) inserted
through the right carotid artery into the left ventricle of the heart
demonstrating typical traces with low diastolic pressure was used
for the injection of microspheres (see below). The catheters were
connected to SensoNor 840 pressure transducers (SensoNor, Horten,
Norway), HP 8805C pressure amplifiers, and a HP 7758A recorder
(Hewlett Packard Company, Waltham, MA).
The abdomen was opened in the midline and an infant blood
pressure gasket (No.1, 3.1 cm to 5.7 cm, Hewlett-Packard, Andover,
MD) was placed around a 250 cm long loop of the distal ileum. A
catheter was inserted into the mesenteric vein proximal to the
pressure gasket and the tip of the catheter was advanced into vein of
the closed bowel loop for continuous recording of venous pressure
and sampling of venous blood. The strangulated bowel loop was
isolated from the abdomen in a plastic bag. A Foley catheter drained
the urinary bladder during the experiment. To compensate for fluid
loss during the operation the animals received Ringer`s acetate 15
mL·hour-1·kg-1 intravenously for the whole operation and stabilisation
period. The animals were allowed 30 min of stabilisation after the
surgical procedure before registration of Baseline variables.
Blood flow and cardiac output
Coloured microspheres (DyeTrak®, Triton Technology, San
Diego, CA) with a diameter of 15 μm and surface coated with a
single dye were used for the measurement of Cardiac Output (CO)
and tissue blood flow. The microspheres were injected into the left
ventricle of the heart over a period of 30 seconds in a number of
approximately 11.5 × 106 for eosin and yellow and 15 × 106 for violet
and blue spheres. The sequence of colours was selected at random.
A reference blood sample was drawn from the femoral artery with
a constant rate extraction pump at a rate of 10 mL·min-1 during
injection of spheres and 90s afterwards. Microspheres for blood flow
and cardiac output measurement were injected at Baseline before
strangulation, after 90 and 180 mins of strangulation, and 25 mins
after release of strangulation obstruction.
The strangulated bowel loop was removed and a segment of
approximately 30 cm was selected for measurement of whole wall
tissue blood flow rate. Tissue samples were also taken from both
kidneys in order to verify homogenous distribution of microspheres
in paired organs. The tissue samples and reference blood samples
were weighed and dissolved overnight in 20 mL of 4 M potassium
hydroxide with 0.05% Tween 80 at 60°C. Each sample was filtered
under vacuum through a 25 mm, 10 μm pore filter (Mitex® Membrane
Filters, Millipore, Ireland). The microspheres were washed with
0.05% Tween 80 and then with ethanol. The filters with their retained
microspheres were centrifuged with 700 μL of dimethylformamide to
elude the dyes. The solution of mixed dyes was scanned photometrical
from 350 nm to 750 nm (Hewlett Packard 8452 A, Diode Array
Spectrophotometer). The spectra obtained were quantified using
partial least square single component analysis on commercial
software (Advanced Chemstation Software, Hewlett Packard). A
small segment of the strangulated bowel was weighed before and
after being dried in an incubator in order to determine the tissue
water content. The tissue blood flow rate expressed as mL·min-1·g-1
dry weight was computed according to standard formula [14]. The
perfusion pressure in the strangulated bowel loop was calculated as
the A-V pressure difference between the femoral artery and the vein
of strangulated bowel.
Blood samples
Blood samples were collected from the femoral artery and
the catheter in the vein of the strangulated bowel loop, just before
induction of strangulation, at 90 and 180 mins of strangulation, and 2
and 25 mins after termination of the strangulation obstruction.
Lactate was analysed in plasma from blood collected in 5 mL
containers with 20 mg of fluoresced heparin (BD Vacutainer,
Belliver Industrial Estate, Plymouth, UK). The blood samples were
immediately chilled, and the plasma was separated from cells within
15 mins in a chilled centrifuge (Megafuge 1.0R, Heraeus Instruments
GmbH, Hanau, Germany). Samples with gross haemolysis were
discarded. Lactate was quantified in an auto-analyser (aca® discrete
clinical analyser, Du Pont Company, Wilmington, DE) by the
Marbach and Weil method, which employs the oxidation of lactate to
pyruvate, assay range 0 mmol.L-1 - 15 mmol.L-1. Arterial and venous
blood pH, pCO2, and pO2 were analysed immediately by an automatic
blood gas analyser system and Base Excess (BE) was calculated (AVL
995-Hb, Graz, Austria).
Histology
Biopsies for histopathological examinations were obtained from
the strangulated bowel loops after 180 mins of strangulation and after
completion of experiments 25 mins after release of strangulation. The
whole-wall tissue samples were kept in Bouin’s solution (750 mL Picric
acid, saturated aqueous solution, 250 mL 37% to 40% formaldehyde,
50 mL Glacial acetic acid) and stained with Haematoxylin-Eosin
(H&E). Microscopic slides were coded and evaluated without
revealing animal identity for the examiner. Intestinal tissue damage
was semi-quantified as follows: Grade 0 = no damage to intestinal
villi, Grade 1 = epithelial damage limited to distal half of the intestinal
villi and Grade 2 = epithelial damage affecting more than distal half
of the intestinal villi. Percent of grade 0, grade 1, and grade 2 damage
were noted for each slide. Demonstration of the different grades of
mucosal damage is published earlier [11].
Cell proliferation was evaluated in sections immunohistochemically
stained for MIB-1 (Ki-67) and counterstained with Haematoxylin-
Eosin (H&E). High power fields and intensely stained nuclei were
chosen for MIB-1 counting. The mean number of proliferating cells
per mm of mucosa was estimated.
Strangulation and reperfusion
The strangulation obstruction was initiated by inflation of the
gasket until the venous pressure of the intestinal loop reached 50
mmHg. By adjusting the gasket pressure, the venous pressure was
kept at 50 mmHg for the first 15 min of obstruction. Thereafter,
gasket pressure was not altered independent of changes in venous
pressure. A short segment of the strangulated intestine was resected
just before termination of strangulation. The intestine was divided
by TLC-55 Linear Cutters, Blue/Regular cartridge (Ethicon Endo-
Surgery, LLC Johnson & Johnson, Guaynabo, Puerto Rico) and the
corresponding mesentery was ligated. Strangulation obstruction was
terminated after 185 mins by relieving the pressure and removing the
gasket from the strangulated intestine. Haemodynamic variables and
blood samples were obtained from systemic arterial blood and the
mesenteric vein of the strangulated intestine 5 mins before, and after
90 and 180 mins of strangulation. Another set of measurements and
blood were sampled 2 and 25 mins after termination of strangulation
obstruction.
Experimental groups
The animals were allocated at random into one of three
experimental groups with eight animals in each group.
Group I (Standard infusion) received infusion of Ringer`s
acetate at a constant rate of 15 mL·kg-1·h-1 during all phases of the
experiment including the period of strangulation and after release
of strangulation obstruction in order to compensate for loss of fluid
related to the basal metabolism and laparotomy.
Group II (High infusion) received infusion of Ringer`s acetate at
a rate of 15 mL·kg-1·h-1 during surgery and stabilisation and thereafter
at a rate of 55 mL·kg-1·h-1 during strangulation obstruction and after
release of strangulation. The enhanced fluid administration intended
to compensate for loss of fluid related to the basal metabolism,
laparotomy and strangulation obstruction.
Group III, the control group (Sham) was operated exactly as
the two intervention groups but the gasket was not inflated. Thus,
strangulation obstruction was not induced. Infusion of Ringer`s
acetate was kept at a constant rate of 15 mL·kg-1·h-1 and evaluated the
ability of base fluid infusion to compensate for fluid losses related to
basal metabolism and laparotomy.
Ethics
The experiment was performed according to “Principles of
laboratory animal care” [12] and the experimental animal board of
the Norwegian Department of Agriculture approved the protocol.
Approval number 200003. The responsible laboratory veterinarian
supervised the experiments under the surveillance of the Norwegian
Animal Research Authority. At the end of an experiment the animal
was sacrificed with an intra-cardiac injection of 20-mL potassium
chloride while still in the same narcosis.
Statistics
The IBM SPSS Statistics ver.20 was used for the statistical analyses.
Fluid administration (Standard or High) during the experiment was
the intervention. Results are presented as mean with Standard Error
of the Mean (SEM) unless stated otherwise. The degree of mucosal
damage, arterial blood gases, blood flow rate and lactate were studied
by two-way ANOVA for repeated measurements (RM-ANOVA)
with Sham, Standard fluid and High fluid as grouping factor (Pb)
and time as within factor (Pw). If the Mauchly’s test of sphericity
was significant, the p-value with a Greenhouse-Geisser adjustment
of the degrees of freedom was noted. The interaction effect (Pi) was
considered significant if p<0.10. In cases with significant interaction
effect, differences between cell means were considered significant
if 95% confidence intervals did not overlap. Otherwise, post hoc
contrast tests between mean values were performed with the Tukey’s
multiple comparison tests and p<0.05 was considered significant.
Table 1
Table 1
Heart rate, mean arterial blood pressure, and cardiac output during strangulation and termination of strangulation obstruction.
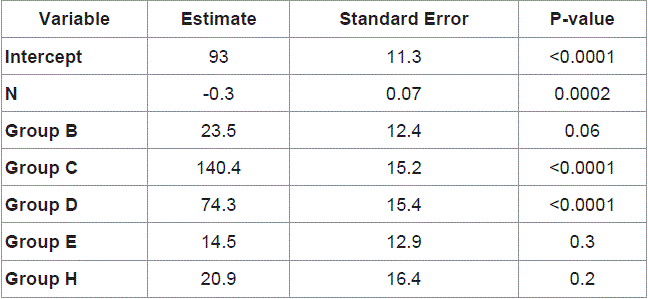
Figure 1
Figure 1
Circulatory changes in the bowel loop during strangulation obstruction and release of strangulation.
Upper panel: mean blood pressure in vein of strangulated loop; middle panel: perfusion pressure across vessels in strangulated loop (Part - Pvein).
Lower panel: tissue blood flow rate. Bars are SEM. Pw, Pi and Pb denotes p-values from the RM-ANOVA for within group, interaction effect and between treatment
groups, respectively.
† p<0.05, different from Sham group * p<0.05, within group change from previous measurement § p<0.05, different from the other intervention group.
Results
The hemodynamic consequences of small bowel strangulation
and subsequent strangulation release are summarised in (Table 1).
Briefly, standard infusion rate of Ringer acetate during strangulation
obstruction was followed by increased heart rate, and a reduction of
arterial blood pressure and cardiac output. Release of strangulation
obstruction killed two of the animals. In the six surviving animals
the arterial blood pressure and cardiac output decreased further. The
hemodynamic of the sham group and the high volume group of fluid
substitution were largely unaffected by strangulation and release of
strangulation.
The blood pressure in the vein draining the bowel loop increased
and remained stable at means of 38.5 mmHg to 46.5 mmHg in the
intervention groups during strangulation. Release of strangulation
reduced the venous blood pressure to baseline levels at means of 9
mmHg to 12 mmHg (Figure 1, upper panel).
Perfusion pressure across the strangulated bowel loop (Figure
1, middle panel) decreased significantly during strangulation in
both intervention groups to means of 18 mmHg to 28 mmHg, when
compared to both baseline and sham operated animals (range of
means: 59 mmHg to 62 mmHg). Animals with standard rate of fluid
administration experienced the lowest perfusion pressure in the
strangulated loop and the perfusion pressure remained low upon
release of strangulation. The high infusion group demonstrated an
increase of perfusion pressure towards baseline and sham group of
animals upon release of strangulation.
There was a clear reduction in tissue blood flow during bowel
strangulation in both intervention groups from baseline averages of
2.9 ml·min-1·g-1 - 3.8 ml·min-1·g-1 to 0.3 ml·min-1·g-1 - 0.9 ml·min-1·g-1
(Figure 1, lower panel). Upon release of strangulation, the blood flow
remained very low in the strangulated bowel loop of the standard
infusion group, whereas the tissue blood flow increased towards
baseline levels in the high infusion group.
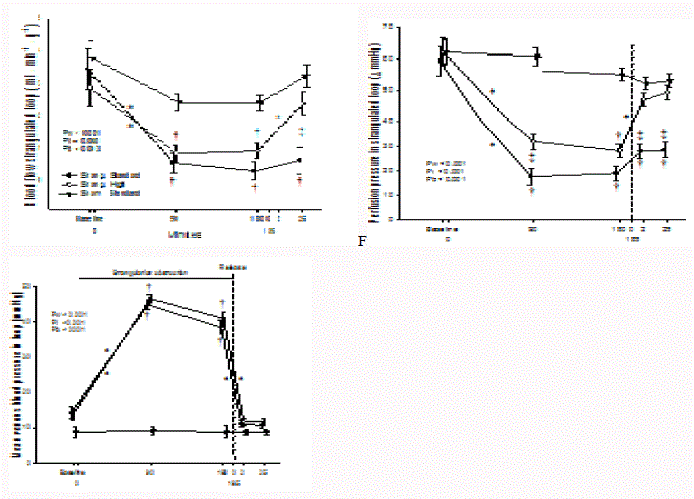
Venous partial pressure of oxygen (pvO2) decreased in the bowel
loop during strangulation from a baseline of 5.9 kPa to 6.1 kPa to
a level of 3.1 kPa to 3.6 kPa in both intervention groups (Figure 2,
upper panel). Release of strangulation improved blood pvO2 within
minutes towards baseline levels in the high infusion group, whereas
pvO2 remained lower in the standard infusion group. Changes in
pvO2 of sham-operated animals were statistically insignificant.
Lactate concentrations from the vein in the strangulated
loop increased significantly from a baseline of 1.7 mmol.L-1 to 2.5
mmol.L-1 towards a level of 5.7 mmol.L-1 to 7.5 mmol.L-1 in both
intervention groups (Figure 2, middle panel). Release of strangulation
rapidly reduced venous lactate level in animals with high volume
administration but still the level was higher than in controls after
2 and 25 mins. In animals with standard fluid administration,
the lactate concentration remained at a level of 7.7 mmol.L-1 - 9.2
mmol.L-1 and significantly higher than in the control and in the high
infusion group.
The venous pH decreased during strangulation from mean
baseline levels of 7.48 to 7.52 to a level of 7.25 to 7.29 in both
intervention groups (Figure 2, lower panel). After release of
strangulation the venous pH remained low in standard infusion
group (pH: 7.19 to 7.29) and increased somewhat in the high infusion
group to an intermediate level (pH 7.35 to 7.4) statistically different
from both the control and standard infusion group.
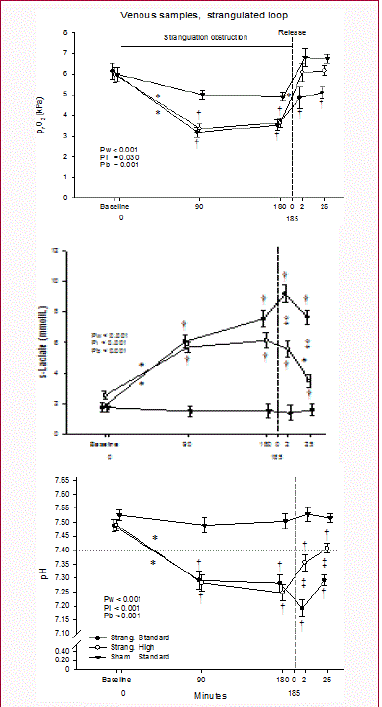
Results of arterial blood samples are summarised in (Figure 3).
A statistical increase in serum lactate level during strangulation and
even more after release of strangulation was noticed in the standard
infusion group, only (Figure 3 upper panel).
Base excess decreased gradually during the experiment from
baseline levels of 7.3 mmol.L-1 to 9.8 mmol.L-1 and remained
significantly lower than in sham-operated controls during
strangulation (5.0 mmol.L-1 to 3.5 mmol.L-1 and 5.5 mmol.L-1 to 2.8
mmol.L-1) and following release of strangulation (2.9 - 3.1 and - 0.4 -
1.1) both in animals with high and standard infusion rate (Figure 3,
middle panel). Changes in arterial pH were modest, but a statistically
significant decrease was noticed by the end of strangulation and after
release of strangulation (within main effect) (Figure 3, lower panel).
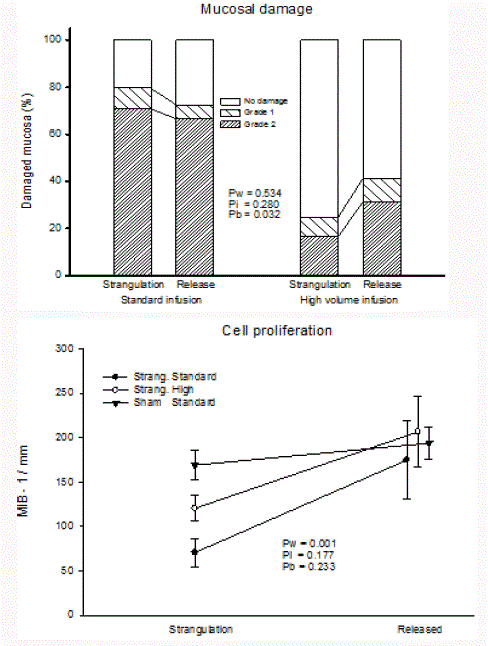
The strangulated bowel had macroscopic signs of severe damage
with intestinal oedema and a bluish discolouration of varying intensity
in both intervention groups. The microscopy results of bowel mucosa
is summarised in (Figure 4). The sham-operated control group was
omitted from the analyses due to absence of mucosal damage. In
biopsies obtained after 180 min of strangulation, mucosal damage in
the standard infusion group was extensive (80% ± 13% of examined
mucosa) when compared to the high infusion group (25% ± 6%).
After release of strangulation, 72% ± 17% and 41% ± 15% of the
mucosa was damaged in the standard and the high infusion groups,
respectively. Despite an apparent numeric increase of grade 2 damage
after release of strangulation in the high infusion group, release of
strangulation produced no statistical increase in mucosal damage.
Over all, the mucosal damage was more pronounced in the standard
than in the high infusion group (p<0.032).
The average number of MIB - 1 stained cell was low in the mucosa
during strangulation (Figure 4, lower panel). The number of MIB
- 1 cells was also statistically lower in the standard infusion group
(71 mm-1 ± 16 mm-1) than in the sham group (169 mm-1 ± 17 mm-
1) (p<0.05 by Tukey HSD). The high infusion group demonstrated
an intermediate level of MIB - 1 stained cell (120 mm-1 ± 16 mm-1).
Release of strangulation increased significantly the number of MIB - 1
stained cells towards the level in the sham group.
Figure 2
Figure 2
Mesenteric vein levels of lactate, Base Excess and pH during
strangulation and release of strangulation obstruction. Bars are SEM.
Upper panel: partial pressure of oxygen in venous blood, pvO2 (kPa); middle
panel: s-Lactate (mmol/L); lower panel: blood pH.
† p<0.05, different from Sham group
* p<0.05, within group change from previous measurement
§ p<0.05, difference between intervention groups
Figure 3
Figure 3
Arterial blood levels of lactate, Base Excess and pH during
strangulation and release of strangulation obstruction.
Upper panel: s-lactate concentration (mmol/L); middle panel: blood Base
Excess; and lower panel: blood pH. Bars are SEM.
† p< 0.05, different from Sham group
§ p<0.05, difference between intervention groups
* p<0.05, within effect, with given levels different from that of 90 minutes of
strangulation
Figure 4
Figure 4
Mucosal damage and cell proliferation in bowels of pigs after 180
min of strangulation and 25 min after release of strangulation.
Upper panel: Percent of grade 0, grade 1, and grade 2 mucosal damage
Lower panel: Cell proliferation identified by MIB-1.
Discussion
This study shows that increased crystalloid fluid administration
improves central haemodynamic and perfusion pressure in a
strangulated bowel segment and protects against mucosal damage
during strangulation and release of strangulation obstruction. Release
of strangulation imposes no additional damaging effect on the bowel
mucosa. Instead, signs of cell proliferation increase immediately as an
indication of viable bowel and early onset of restitution.
Signs of anaerobe metabolism in peripheral arterial blood are
modest and only noticed in case of insufficient volume substitution.
Release of strangulation obstruction under such circumstances may
be deleterious as two animals succumbed immediately.
Hemodynamic/anaerobe metabolism
The standard infusion rate of Ringer`s acetate is sufficient to
compensate for fluid loss due to basal metabolism and laparotomy
throughout the experiment in the control group (Table 1). The
hemodynamic changes during strangulation are therefore most likely
a consequence of excess fluid loss during strangulation [3]. Insufficient
volume substitution with hypovolemia renders the animals susceptible
to vasodilation, drop in blood pressure and even death after release
of strangulation (Table 1). The effect of insufficient crystalloid
substitution on the hemodynamic is particularly clear upon release of
strangulation (Figure 1). Experiments with strangulation obstruction
in rats suggest infusion of hypertonic saline is superior to identical
volume of Ringer lactate [13]. Hypertonic saline effectively mobilize
cellular water into the blood volume and the volume expansion by
hypertonic saline may reach 10 times of what is obtained by lactated
Ringer's solution [14]. Sufficient substitution of fluid with crystalloids
is therefore important to avoid hemodynamic changes during both
strangulation and release of strangulation obstruction.
The mechanisms
Bacterial translocation occurs even in simple intestinal
obstruction in humans [15]. In experiments, high weight hydrophilic
marker molecules continue to translocate from bowel to venous
blood after release of strangulation and during restitution of mucosal
damage as indication of a continuous barrier deficit in strangulation
obstruction [4]. Bacterial translocation is not evaluated in this study
but hypertonic saline as volume substitution significantly reduce
bacteraemia in rats [13]. This may be one of the effects of sufficient
volume substitution in strangulation obstruction.
Although strangulation of bowel facilitates bacterial translocation,
other substances from the strangulated bowel may modify vascular
tone upon release of strangulation. Distinct traces of anaerobe
metabolism in peripheral arterial blood and hypotension after release
of strangulation in the standard volume substitution group (Figure
3, Table 2) suggest a vicious circle of prolonged low flow state with
anaerobe metabolism, acidosis, or release of vasoactive substances
from the strangulated bowel [16,17]. The effect of such substances is
probably modest since high volume substitution easily prevents most
of the effects (Figure 1 and 2, Table 1).
Mucosal/Bowel damage
There is a striking difference in mucosal damage between the
standard and the high volume infusion group (Figure 4) which is
related to mucosal blood flow [10] although differences in perfusion
pressure and blood flow are modest (Figure1 and 4). Improved blood
flow alone is therefore not a satisfactory explanation of reduced
mucosal damage in the high infusion group. The parallel reduction
in pvO2 and pH, and increase of lactic acid in the mesenteric vein
during strangulation obstruction in the two groups (Figure 2) are
also somewhat inconsistent with different degree of mucosal damage.
Thus, both intestinal blood flow and metabolic changes detected in
the mesenteric vein during strangulation obstruction seem unable to
predict the degree of intestinal damage. A modulation of leukocyte
endothelial interactions by hypertonic saline described by Luiz
Zanoni et al. [13] May be the missing link in the explanation of
reduced bowel damage in the high infusion group.
The majority of the mucosal damage associated with ischemia
occurs during reperfusion and not during the ischemic period
[6]. Reperfusion by release of strangulation inflicts no additional
damage to the mucosa within the first 25 mins (Figure 4). Laws et
al. [18] notice similar results. The already extensive mucosal damage
induced by strangulation obstruction seen in the standard infusion
group suggests that any additional or progressive mucosal damage
by release of strangulation may be impossible to identify. The slight
but statistically insignificant increase of grade 2 damage after release
of strangulation in high volume group indicates that some mucosal
damage may occur upon release of strangulation, but not to the
extent seen in reperfusion after an arterial occlusion. Thus, further
mucosal or bowel damage should not be expected upon release of
strangulation obstruction.
It can be argued that the time of reperfusion (25 mins) in this
study is short compared to that of experiments with complete arterial
occlusion demonstrating mucosal damage after the 60 mins of
reperfusion [6]. However, suppressed cell proliferation as evaluated
by MIB - 1 is already reactivated to control levels 25 mins after release
of strangulation (Figure 4) and strangulation studies in horse bowel
show no changes 90 mins after release of venous occlusion [18].
Moreover, 4-hrs after release of strangulation the bowel mucosa
approaches complete restitution [4]. Thus, a relative short observation
time from release of strangulation seems sufficient for the detection of
early signs of reperfusion damage and restitution in damaged bowel
mucosa.
Restitution is time consuming and involves migration of cuboid
cells along the basement membrane towards the tip of the villi [19],
and is strongly associated with the extent of strangulation [4]. High
volume fluid substitution may therefore reduce mucosal damage,
predispose to expeditious recovery of the mucosa and reduce bacterial
translocations from the bowel to the blood [4,13].
Clinical implications
Parameters contributing to the identification of strangulation
obstruction are of great interest in non-operative management
and triage of small bowel obstruction by for instance water-soluble
contrast [20]. Decline in peripheral blood level of Base Excess (BE)
occurs during strangulation obstruction and identify metabolic
acidosis at a level that may be consistent with reversible bowel ischemia
(Figure 3). This is a new observation, since earlier studies show that
BE reduction is associated with bowel gangrene and bowel resection
with a sensitivity and specificity of 75% and 80%, respectively [21,22].
Lactate in peripheral blood is also a marker of nonviable
bowel strangulation [23,24]. However, release of lactic acid to the
mesenteric vein during strangulation (Figure 2) is easily masked in
peripheral blood by extensive crystalloid fluid administration (Figure
3). Elevated lactic acid in peripheral blood may therefore characterize
the general circulatory status rather than the anaerobe metabolism of
a strangulated bowel. Although not easily available, peritoneal fluid
lactate may be more precise in detection of intestinal strangulation
and abdominal catastrophes [25]. The modest changes in arterial pH
seen in the present study are consistent with the poor predictive value
of peripheral blood pH in clinical studies [22].
The hazard of fluid loss, intravascular volume depletion, and
hemodynamic changes (Table 1) is probably related to systemic
effect of metabolic acidosis or other factors released from the
bowel both during and following the release of strangulation [3]
[26-28]. The effect of substances be from the strangulated bowel is,
nevertheless, modest as crystalloid fluid administration alone appears
to compensate for the effect (Table 1). Sufficient fluid administration
cannot be overrated in strangulation obstruction as it enables the pigs
to withstand changes in vascular tone, cardiac function, and death.
The recovery of suppressed cell proliferation to control level
within 25 mins of release from strangulation (Figure 4) suggests
that the strangulated bowel may be viable and the bowel should be
assessed for preservation. Clinically, an attitude towards conservation
of bowel and second look strategies may be justified in situations with
risk of extensive bowel loss.
Metabolic changes detected in mesenteric vein of the two
intervention groups’ reveal similar reductions in pO2 and pH, and
a similar rise in lactic acid during strangulation obstruction (Figure
2). Thus, an increase in oxygen extraction and anaerobe metabolism
during surgery for strangulation obstruction is unable to predict the
degree of mucosal and bowel damage. Intraoperative near-infrared
fluorescence angiography predicts survival of ischaemic bowel with
greater accuracy than clinical evaluation in animal experiments
[29]. This technique may also prove helpful in predicting viability of
strangulated bowel in the future.
Experiment evaluation
Changes in sham-operated animals (Figure 1 and 2) are probably
due to handling and positioning of the un-inflated gasket around the
bowel segment. Nevertheless, the lack of bowel damage in the control
group confirms that standard infusion rate of Ringer`s acetate is
sufficient to compensate for fluid loss due to basal metabolism and
laparotomy in this experimental model.
A single point of evaluation after release of strangulation may
inflict study limitations. However, the rapid improvements of
blood flow (Figure 1), metabolism (Figure 2), hemodynamic, and
suppressed cell proliferation encourage early evaluation upon release
from strangulation. Similarly, Juel et al. [4] show that hemodynamic
and tissue blood flow returns close to baseline and few changes
are observed in these parameters beyond the first hr of release
from strangulation [4]. Moreover, restitution of ischemic mucosa
commence within 60 mins of reperfusion [30] and restitution of
damaged mucosa is well in progress with villi covered by normal
columnar or cuboidal cells four hours after release of strangulation
[4]. Thus, evaluation of reperfusion damages in strangulation
obstruction must occur very early.
Conclusion
Careful observation for hypotension, tachycardia and biochemical changes related to metabolic acidosis may contribute to early recognition of intestinal strangulation obstruction. Reperfusion damages in the strangulated bowel should not be expected upon release of strangulation. Enhanced intravenous fluid administration during preparation to operation and during the surgical procedure may reduce bowel damage and hemodynamic consequences of strangulation obstruction.
Acknowledgment
This study was supported by the Research Council of Norway (P.No. 111484/320).
References
- Fevang BT, Fevang J, Stangeland L, Soreide O, Svanes K, Viste A. Complications and death after surgical treatment of small bowel obstruction: A 35-year institutional experience. Ann Surg. 2000;23(4):529-37.
- Fevang BT, Jensen D, Svanes K, Viste A. Early operation or conservative management of patients with small bowel obstruction? Eur J Surg. 2002;168(8-9):475-81.
- Fevang J, Fevang BT, Gislason H, Svanes K. Hemodynamic changes associated with strangulation obstruction in cats. Int J Microcirc Clin Exp. 1995;15(6):325-30.
- Juel IS, Solligard E, Skogvoll E, Aadahl P, Gronbech JE. Lactate and glycerol released to the intestinal lumen reflect mucosal injury and permeability changes caused by strangulation obstruction. Eur Surg Res. 2007;39(6):340-9.
- Thompson JS, DiBaise JK, Iyer KR, Yeats M, Sudan DL. Postoperative short bowel syndrome. J Am Coll Surg. 2005;201(1):85-9.
- Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986;250(6):G749-53.
- Chu W, Li S, Wang S, Yan A, Nie L. Ischemic post conditioning provides protection against ischemia-reperfusion injury in intestines of rats. Int J Clin Exp Pathol. 2015;8(6):6474-81.
- Santos CH, Gomes OM, Pontes JC, Miiji LN, Bispo MA. The ischemic preconditioning and postconditioning effect on the intestinal mucosa of rats undergoing mesenteric ischemia/reperfusion procedure. Acta Cir Bras. 2008;23(1):22-28.
- Freeman DE, Cimprich RE, Richardson DW, Gentile DG, Orsini JA, Tulleners EP, et al. Early mucosal healing and chronic changes in pony jejunum after various types of strangulation obstruction. Am J Vet Res. 1988;49(6):810-8.
- Fevang J, Ovrebo K, Grong K, Svanes K. Fluid resuscitation improves intestinal blood flow and reduces the mucosal damage associated with strangulation obstruction in pigs. J Surg Res. 2004;117(2):187-194.
- Fevang J, Ovrebo K, Svanes K, Rokke O. Endotoxin and cytokine release in strangulation obstruction and in partial occlusion of the mesenteric artery in pigs. Eur Surg Res. 1999;31(1):26-38.
- Laboratory CftUotGftCaUo, Council ANR: Guide for the Care and Use of Laboratory Animals. Eighth Edition. Washington, D.C. : National Academies Press; 2011.
- Luiz ZF, Costa Cruz JW, Martins JO, Benabou S, Vicente GK, Ramos Moreno AC, et al. Hypertonic saline solution reduces mesenteric microcirculatory dysfunctions and bacterial translocation in a rat model of strangulated small bowel obstruction. Shock. 2013;40(1):35-44.
- Kramer GC. Hypertonic resuscitation: physiologic mechanisms and recommendations for trauma care. J Trauma. 2003;54(5):S89-99.
- Deitch EA. Simple intestinal obstruction causes bacterial translocation in man. Arch Surg. 1989;124(6):699-701.
- Amundsen E, Midtvedt T. The toxicity of fluid from experimentally strangulated intestinal loops in the rat. J Surg Res 1964;26(4):306-13.
- Hattori K, Tsuchida S, Tsukahara H, Mayumi M, Tanaka T, Zhang L, et al. Augmentation of NO-mediated vasodilation in metabolic acidosis. Life Sci. 2002;71(12):1439-47.
- Laws EG, Freeman DE. Significance of reperfusion injury after venous strangulation obstruction of equine jejunum. J Invest Surg. 1995;8(4):263-70.
- Svanes K, Ito S, Takeuchi K, Silen W. Restitution of the surface epithelium of the in vitro frog gastric mucosa after damage with hyperosmolar sodium chloride. Morphologic and physiologic characteristics. Gastroenterology. 1982;82(6):1409-26.
- Branco BC, Barmparas G, Schnuriger B, Inaba K, Chan LS, Demetriades D. Systematic review and meta-analysis of the diagnostic and therapeutic role of water-soluble contrast agent in adhesive small bowel obstruction. Br J Surg. 2010;97(7):470-8.
- Sarr MG, Bulkley GB, Zuidema GD. Preoperative recognition of intestinal strangulation obstruction. Prospective evaluation of diagnostic capability. Am J Surg. 1983;145(1):176-82.
- Takahashi R, Akagi Y, Tanaka T, Kaibara A, Kajiwara S, Shima I, et al. Clinico pathological evaluation of anoxic mucosal injury in strangulation ileus. BMC Surg. 2014;14:79.
- Tanaka K, Hanyu N, Iida T, Watanabe A, Kawano S, Usuba T, et al. Lactate levels in the detection of preoperative bowel strangulation. Am Surg. 2012;78(1):86-8.
- Tanaka K, Hashimoto H, Ohki T. Lactate levels in bowel strangulation with experimental animal model. Int Surg. 2015;100(2):240-3.
- Latson KM, Nieto JE, Beldomenico PM, Snyder JR. Evaluation of peritoneal fluid lactates as a marker of intestinal ischaemia in equine colic. Equine Vet J. 2005;37(4):342-6.
- Aalkjaer C, Poston L. Effects of pH on vascular tension: which are the important mechanisms? J Vasc Res 1996;33(5):347-59.
- Wang X, Wu J, Li L, Chen F, Wang R, Jiang C. Hypercapnic acidosis activates KATP channels in vascular smooth muscles. Circ Res. 2003;92(11):1225-32.
- Zhou HZ, Malhotra D, Shapiro JI. Contractile dysfunction during metabolic acidosis: role of impaired energy metabolism. Am J Physiol. 1991;261(5):H1481-H1486.
- Matsui A, Winer JH, Laurence RG, Frangioni JV. Predicting the survival of experimental ischaemic small bowel using intraoperative near-infrared fluorescence angiography. Br J Surg. 2011;98(12):1725-34.
- Derikx JP, Matthijsen RA, de Bruine AP, van Bijnen AA, Heineman E, van Dam RM, et al. Rapid reversal of human intestinal ischemia-reperfusion induced damage by shedding of injured enterocytes and reepithelialisation. PLoS One. 2008;3(10):e3428.