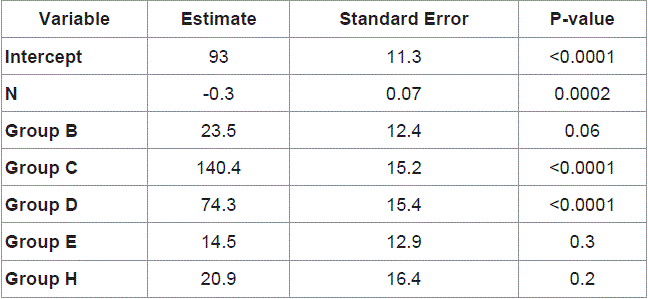
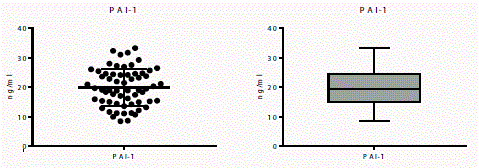Research Article
Synovial Fluid Analysis in Patients with Osteoarthritis Requiring Total Joint Arthroplasty
Elissa S. Davis1*, Christopher Wanderling2, Daniel R. Schmitt1, Jeffrey Liles2, Kevin Galicia2, Debra Hoppensteadt3, Jawed Fareed3 and William Hopkinson1
1Department of Orthopaedic Surgery and Rehabilitation, Loyola University Medical Center, USA
2Loyola University of Chicago, Stritch School of Medicine, Maywood, IL
3Department of Pathology, Loyola University Medical Center, USA
*Corresponding author: Elissa S Davis, Department of Orthopaedic Surgery and Rehabilitation, Loyola University Medical Center, 2160 S 1st Ave, Maywood, IL, 60153, USA
Published: 18 Jul, 2018
Cite this article as: Davis ES, Wanderling C, Schmitt DR, Liles J, Galicia K, Hoppensteadt D, et al. Synovial Fluid Analysis in Patients with Osteoarthritis Requiring Total Joint Arthroplasty. World J Surg Surgical Res. 2018; 1: 1025.
Abstract
Background: Osteoarthritis (OA) is a progressive disease characterized by degeneration of the articular cartilage in joints. Currently, there are no preventative treatments available for OA, which has resulted in an increasingly heavy burden on the country’s healthcare system as the population continues to age. Despite the increasing emphasis on preventative health care overall in medicine, there have been minimal recent improvements in the prevention or management of OA.
Objective: To profile total protein content, CRP, IL-1b, MP-TF and PAI-1 in patients who suffer from OA that require Total Joint Arthroplasty (TJA) procedure.
Method: SF samples were taken perioperatively from 62 patients undergoing either TKA or THA procedures. The SF was then purified by centrifugation. Samples were aliquoted and stored at 80°C. Samples were analyzed for total protein by photo spectrometry and ELISA assays were used to analyze CRP, IL-1b, MP-TF and PAI-1 levels.
Results: The mean total protein load was 19.27 mg/ml ± 13.89 mg/ml. The mean values for these biomarkers were: CRP 1.54 ng/ml ± 1.86 ng/ml, IL-1b 0.19 pg/ml ± 0.18 pg/ml, MP-TF 14.17 pg/ml ± 19.94 pg/ml and PAI-1 19.87 ng/ml ± 6.29 ng/ml.
Conclusion: This novel study investigating the content of SF demonstrates that these biomarkers are present at detectable levels. These results may help better define the pathogenesis of OA and aid in the development of targeted therapies against the inflammatory pathway.
Keywords: Osteoarthritis; Arthroplasty; Synovial fluid; Inflammation
Introduction
Osteoarthritis (OA), also known as Degenerative Joint Disease (DJD), is a progressive disease characterized by the loss of articular cartilage in joints. The prevalence of OA is known to increase with aging, with approximately 20% of the population reporting symptoms related to osteoarthritis at age 45 years or older [1]. The continued aging of our population has resulted in a significant impact on the increasing rates of OA, particularly in the load-bearing joints of the hip and knee [1].
Currently, available treatments for OA are limited to symptom management which has resulted in an increasingly heavy burden on healthcare cost. Losina et al. [2] investigated the financial impact of OA management and found that the average annual cost ranged from $989 to $10,313 per person. Additionally, the authors examined average lifetime costs to patients and reported a guideline-concordant management for symptomatic OA of approximately $12,400 [2].
Despite the increasing emphasis on preventative health care, there have been minimal recent improvements in the prevention or management of DJD. Current first-line interventions for OA are focused on pain management and lifestyle changes; these modalities include physical therapy, NSAID use, and intra-articular corticosteroid injections. While they have been shown to be effective in the temporary management of OA, they do not adequately address the underlying pathophysiology of DJD. As a result, many patients will eventually fail these primary interventions and require a Total Joint Arthroplasty (TJA) procedure [3,4].
Over one million TJA procedures of the hip or knee are performed annually in the US. With continually increasing lifespans, TJA is on pace to become the most commonly performed elective procedure in America-currently there are approximately 2.5 million and 4.7 million individuals living with Total Hip and Total Knee Arthroplasty (TKA and THA) prosthesis, respectively [5]. While TJA of the hip and knee have been shown to be effective in the management of DJD, these procedures are also not without substantial risk of adverse effects, including risks associated with anesthesia, prosthetic joint infection, and need for further revision surgery [6].
While TJA procedures typically provide adequate relief from joint pain, more specific intervention targeting the pathophysiology prior to development of end-stage arthritis requiring arthroplasty may be a novel intervention.
Systemic analysis of serum biomarkers in patients with OA have previously been performed which identified significant results. Serum inflammatory biomarkers such as C-Reactive Protein (CRP) and IL-6 were shown to be correlated with disease severity regarding pain and Kellgren and Lawrence radiographic grade [7,8]. Additionally, serum molecules such as fibrinogen and tissue factor were shown to be elevated which is consistent with states of inflammation [9]. Previously, this lab reported serum levels of inflammatory markers in patients with OA requiring TJA. While the analysis of systemic biomarkers is of value, a more specific investigation into the joint itself may help elucidate the pathogenesis of OA. The purpose of this study is to examine inflammatory markers in Synovial Fluid (SF) samples from patients suffering from OA requiring TKA or THA in order to characterize changes in inflammatory markers, better understand the pathogenesis of OA and identify potential targets for future therapies. Total protein, IL-1b, CRP, Microparticles-Tissue Factor (MP-TF), and Plasminogen Activator Inhibitor (PAI-1) levels are measured in these SF samples because these inflammatory markers have all been shown to be elevated in states of inflammation and have been detected in serum at elevated levels in patients with OA [10-13].
Method
De-identified SF samples were obtained between September 2016 and June 2017 from patients with OA undergoing primary TKA or THA. SF was obtained intra-operatively on the day of surgery from patients who signed informed consent. Patients undergoing TJA revision or TKA/THA for indications other than OA (e.g. post-traumatic arthritis, rheumatoid arthritis, or avascular necrosis) were excluded from this study. The influence of age, sex, BMI, and comorbidities (ex. diabetes) on inflammatory biomarker levels was also examined. A total of 62 specimens (46 TKA, 16 THA) were obtained and all met the pre-screened inclusion criteria and are included in the analysis. The TJA procedures were performed at a single institution by four orthopedic surgeons who were fellowship-trained in joints and reconstruction. Patient SF samples were collected immediately following the intra-operative arthrotomy and immediately transferred into empty tubes. SF samples were then centrifuged at 3,000 RPM for 15 minutes and then supernatant was removed in order to minimize contamination from blood that may have occurred during surgery. In order to reduce repeated freeze-thaw cycles, SF samples were aliquoted into separate tubes. SF samples were stored at 80°C. Protein measurements were performed on the NanoDrop 8,000 spectrophotometer (Thermo scientific, Waltham, Massachusetts, USA). SF samples were analyzed using ELISA kits for IL-1b, CRP, MP-TF, and PAI-1 levels (Hyphen Biomed, Neuville-sur-Oise, France). In addition to reporting individual inflammatory biomarker results, correlation analyses were also performed in order to identify the strength of the relationship between any of the inflammatory markers.
This study was approved by the IRB of Loyola University Chicago Health Sciences Division.
Statistical Analysis
Data was analyzed using Microsoft excel and GraphPad prism software version 6. The results were expressed as mean ± standard deviation. Correlation analysis was carried out using the nonparametric Pearson test. P values less than 0.05 were considered statistically significant.
Results
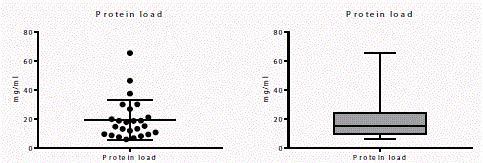
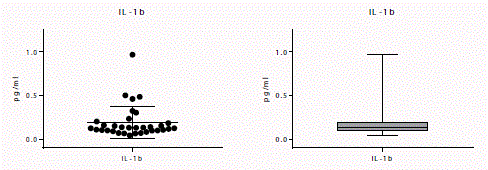
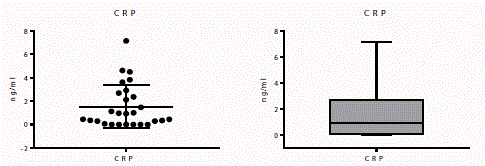
The mean age of the 62 patients included in this study was 64.26 ± 11.91 years. Measured values for the SF samples for total protein, IL-1b, CRP, MP-TF, and PAI-1 are shown in Table 1. The scatter and box plots for total protein and each of the investigated biomarkers are shows in Figures 1-5.
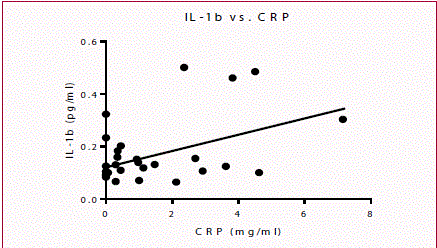
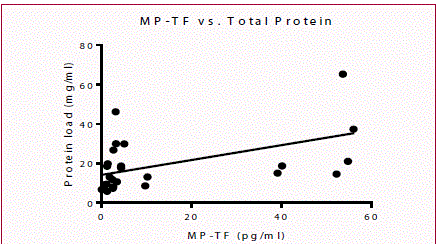
When comparing biomarkers, correlations were identified between IL-1b and CRP (p=0.0238, Pearson r=0.4336) as well as between total protein and MP-TF (p=0.0294, Pearson r=0.4358), which are shown in Figures 6 and 7. No other statistically significant correlations were identified.
Evaluation of the impact of clinical parameters such as age, sex, BMI, comorbidities (ex. diabetes) on biomarkers revealed no significant correlation.
Discussion
The results of this study are consistent with the results of previous studies that have examined SF in patients with OA [14]. Specifically, elevated levels of some synovial fluid inflammatory markers are identified in patients with DJD. The results of IL-1b and CRP in this study are of particular interest.
It has been found that IL-1b has many functions; it promotes the synthesis of degrading enzymes, inhibits synthesis of enzymes that inhibit these degrading proteins, and inhibits synthesis of cartilage matrix constituents such as collagen and proteoglycans. These characteristics of IL-1b result in severe cartilage degradation [15]. Consistent with the functions of IL-1b, one study found that IL-1b levels were directly related to degree of pain and decreased functionality of affected joints [16]. Although this study did not examine IL-1b levels in healthy controls, previous studies investigating Rheumatoid Arthritis (RA) have done so. One study reported a mean value of IL-1b in healthy controls 4.60 pg/ml [17]. Interestingly, the level of IL-1b measured in this OA population was much lower, 0.1898 ± 0.1833 pg/ml.
While a provocative finding initially, these findings may be explained by the burnout hypothesis. Previously described by Kallman et al. [18], the burnout phenomenon is discussed with regards to the Kellgren-Lawrence radiographic scale examining OA- early stage OA may progress to middle stage OA over the course of 10 to 20 years. However, there is a drastically slowed progression to the late stages of OA [18]. It is possible that radiographic evidence of progression of OA may be the result of decreased production of inflammatory biomarkers due to apoptosis or decreased function of chondrocytes or other synovial cells. One study showed results that support this hypothesis- IL-6 levels were present in decreasing levels as the Kellgren-Lawrence score increased [19].
Previous studies have found detectable levels of compounds- including IL-1b on chondrocytes and the synovial membrane [14,20].
CRP has been extensively studied in serum for many inflammatory diseases due to its rapid fluctuation in relation to states of inflammation. As a result, CRP has become a first-line biomarker for detection of inflammation; however it is very non-specific due to its increase related to inflammation in general. As a result, it may be more beneficial to measure CRP levels locally in the SF. While SF analysis of CRP is lacking, it has been studied in serum of patients with OA it was found that CRP levels in serum were elevated in direct relation to disease severity and with Kellgren and Lawrence radiographic scores [8]. One study that did examine the SF of OA patients showed an elevation of high-sensitivity CRP (hsCRP) in the SF of OA patients with inflammatory synovium infiltrates [21]. Although hsCRP is a more sensitive test because it is able to detect CRP at low-level elevations, it is also a more expensive test to perform. This increased cost may not be practical for the purpose of OA detection. While it is not possible to develop any conclusions about CRP specifically at this time, it is important to note that there was a positive correlation between IL-1b and CRP levels. This finding may be a coincidence due to the non-specific nature of IL-1b and CRP in inflammatory states; however potential interaction between these biomarkers cannot be excluded. This interaction can be supported by the previous findings that IL-1 blockade was correlated with lower levels of CRP (and IL-6 another marker of inflammation), although this finding was demonstrated in autoinflammatory disorders [10].
PAI-1, a non-specific acute-phase reactant similar to CRP, is routinely studied. It is elevated in many inflammatory disorders and was found to be significantly elevated in the serum of patients with OA [22]. Previously, PAI-1 has been measured in the SF of patients with OA and the median level was measured at 65.84 ng/ml while the median level for healthy controls was 27.8 ng/ml [23]. Compared with the present study, the mean level for the OA patients was 19.87 ng/ml (median 19.32 ng/ml). The results of this study are drastically different from those of the previous study. This may be attributable to the variability in patient populations the previous study did not specify for disease severity while this study focused on patients with end-stage OA requiring TJA. While the measured values are different, this distinction may be explained by the burnout phenomenon. Further investigation focusing on PAI-1 levels in SF in patients with varying stages of OA severity may help to further elucidate the impact of burnout phenomenon on levels of inflammatory markers in SF, including PAI-1.
MP-TF, commonly associated with vascular and thrombotic disorders, has previously been shown to be elevated, although not significantly, in the serum of patients with OA. While this was not a significant change when compared to healthy controls, tissue factor was significantly decreased when compared in the same patient population [9]. Microparticles have previously been studied in SF of patients with RA; however MP-TF was not specifically studied. Since there is a paucity of data regarding MP-TF in OA, specifically in SF, this may be a novel focus in the future for further investigation into the pathophysiology of OA.
While the significance of MP-TF in SF is yet to be determined, one interesting finding is the positive correlation between MP-TF and total protein level, as shown in Figure 7. A possible explanation for this correlation may be related to the multi-step theory of OA pathogenesis. As the disease progresses, cartilage and synovium degradation continue to release inflammatory debris into the SF, including MP-TF. Many of these inflammatory mediators are phagocytosed by the synovial cells while MP-TF, among other proteins, are not able to be phagocytosed, thus remain suspended in SF and are present at detectable levels [15]. It may be of interest to stratify disease severity with the measured levels of MP-TF, to determine the relationship between MP-TF and OA severity, particularly in patients who have reached the burnout phase of OA, as determined by the levels of inflammatory markers in SF.
Table 1
Figure 1
Figure 1
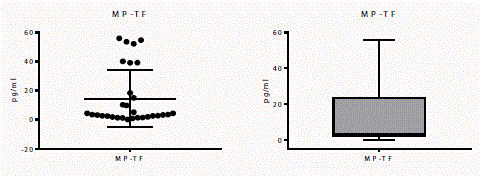
(a) Scatter plot, (b) Bar graph of total protein load measurement, mean value of 19.27 mg/ml ± 13.89 mg/ml.
Figure 2
Figure 2
(a) Scatter plot, (b) Bar graph of IL-1b measurement, mean value of 0.1898 pg/ml ± 0.1833 pg/ml.
Figure 3
Figure 4
Figure 4
(a) Scatter plot, (b) Bar graph of MP-TF measurement, mean value of 14.47 ng/ml ± 19.49 pg/ml.
Figure 5
Figure 5
(a) Scatter plot, (b) Bar graph of PAI-1 measurement, mean value of 19.87 ng/ml ± 6.291 ng/ml.
Figure 6
Figure 7
Conclusion
This study demonstrates that measurable levels of inflammatory biomarkers are present in synovial fluid of patients with OA undergoing TJA. Furthermore, these results may help better define the pathogenesis of OA, particularly when considering the correlation findings between CRP and IL-1b and MP-TF and total protein. Confirming these results and comparing to healthy controls may lead to the development of specific treatment modalities targeting the inflammatory pathway early in the development of OA thus obviating or delaying the need for surgical intervention.
Limitations
Given the prevalence of degenerative joint disease in the population, finding an adequate age-matched control group is exceedingly difficult. Any age-matched cohort used as a control group would likely have degenerative joint changes even if osteoarthritis was not documented as a medical condition in their medical record or if they were asymptomatic, resulting in an imperfect control group at best. Therefore, while there is no control group for this study, the finding that these biomarkers are present at measurable, thus comparable, levels should not be underestimated. Additionally, the variation in the number of subjects examined for each biomarker can be attributed to the variability in volume of SF that was obtained from patients in addition to the analyses being performed concurrently with sample collection. Future studies examining the SF from healthy, age-matched controls along with concomitant study of SF and plasma may clarify the findings identified in this study.
Acknowledgement
The authors gratefully acknowledge the technical support from the staff of the Loyola University Chicago laboratory for thrombosis and hemostasis. We also greatly appreciate the assistance of members of the Department of Orthopaedic Surgery and Rehabilitation for facilitating the logistics of the SF collection, storage, and analysis of the samples included in this study-including Dr. Wu, Dr. McAsey and Dr. Rees along with the resident physicians for their support and encouragement during this study. We are also thankful to the Departments of Pathology and Hemostasis at Loyola University Chicago for providing support and resources to conduct this study. None of the authors who participated in this study had any professional and financial affiliations.
Contribution
All authors have made substantial contribution to the conception and design of the study, drafting and revising of this submission and final approval of the submitted manuscript.
References
- Wallace IJ, Worthington S, Felson DT, Jurmain RD, Wren KT, Maijanen H, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc Natl Acad Sci USA. 2017;114(35):9332-6.
- Losina E, Paltiel A, Weinstein A, Yelin E, Hunter DJ, Chen SP, et al. Lifetime medical costs of knee osteoarthritis management in the United States: Impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken). 2015;67(2):203-15.
- Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;2:CD005328.
- Jones A, Doherty M. Intra-articular corticosteroids are effective in osteoarthritis but there are no clinical predictors of response. Ann Rheum Dis. 1996;55(11):829-32.
- Maradit Kremers H, Larson D, Crowson C, Kremers WK, Washington RE, Steiner CA, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386-97.
- Orthoinfo.aaos.org/en/treatment/total-joint-replacement/.
- Khaltaev N, Pfleger B, Woolf AD, Akesson K, Hazes JM, Symmons D. Health needs assessment of musculoskeletal conditions: an international bone and joint burden of disease study. Global arthritis research network: 4th world congress on arthritis in montreal. Arthritis Res Ther. 2004;6(3):103.
- Sowers M, Jannausch M, Stein E, Jamadar D, Hochberg M, Lachance L. C-reactive protein as a biomarker of emergent osteoarthritis. Osteoarthritis Cartilage. 2002;10(8):595-601.
- Wanderling C, Liles J, Finkler E, Carlsgaard P, Hopkinson W, Guler N, et al. Dysregulation of tissue factor, thrombin-activatable fibrinolysis inhibitor and fibrinogen in patients undergoing total joint arthroplasty. Clin Appl Thromb Hemost. 2017;23(8):967-72.
- Dinarello C. Immunological and inflammatory functions of the interleukin-1 family. Ann Rev Immunol. 2009;27:519-50.
- Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32(4):274-8.
- Distler JH, Pisetsky DS, Huber LC, Kalden JR, Gay S, Distler O. Microparticles as regulators of inflammation: novel players of cellular crosstalk in the rheumatic diseases. Arthritis Rheum. 2005;52(11): 3337-48.
- Aso Y. Plasminogen activator inhibitor (PAI)-1 in vascular inflammation and thrombosis. Front Biosci. 2007;12:2957-66.
- Sowers MR, Karvonen-Gutierrez C. The evolving role of obesity in knee osteoarthritis. Curr Opin Rheumatol. 2010;22(5):533-7.
- Martel-Pelletier J. Pathophysiology of osteoarthritis. Osteoarthritis Cartilage. 2004;12:S31-3.
- Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21(1):16-21.
- Lettesjö H, Nordström E, Ström H, Nilsson B, Glinghammar B, Dahlstedt L, et al. Synovial fluid cytokines in patients with rheumatoid arthritis or other arthritic lesions. Scand J Immunol. 1998;48(3):286-92.
- Kallman DA, Wigley F, Scott WW, Hochberg MC, Tobin JD. The longitudinal course of hand osteoarthritis in a male population. Arthritis Rheum. 1990;33(9):1323-32.
- Orita S, Koshi T, Mitsuka T, Miyagi M, Inoue G, Arai G, et al. Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC Musculoskelet Disord. 2011;12:144.
- Farahat MN, Yanni G, Poston R, Panayi G. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1993;52(12):870-5.
- Pearle AD, Scanzello CR, George S, Mandi LA, DiCarlo EF, Peterson M, et al. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthritis Cartilage. 2007;15(5):516-23.
- Guler N, Burleson A, Syed D, Banos A, Hopkinson W, Hoppensteadt D, et al. Fibrinolytic dysregulation in total joint arthroplasty patients: potential clinical implications. Clin Appl Thromb Hemost. 2016;22(4):372-6.
- Belcher C, Fawthrop F, Bunning R, Doherty M. Plasminogen activators and their inhibitors in synovial fluids from normal, osteoarthritis and rheumatoid arthritis knees. Ann Rheum Dis. 1996;55(4):230-6.