Case Report
Growing Teratoma Syndrome: A Report of a Rare Case with a Detailed Review of the Literature
Mehmet Üstün1*, Avni Can Karaca2, Tayfun Yoldaş3, Taylan Özgür Sezer3 and Cemil Çalışkan3
1Department of General Surgery, Tepecik Training and Research Hospital, University of Health Sciences, Turkey
2Department of General Surgery, Izmir University of Economics, Turkey
3Department of General Surgery, Ege University, Turkey
*Corresponding author: Mehmet Üstün, Department of General Surgery, Tepecik Training and Research Hospital, University of Health Sciences, A Block 1st Floor 1 Surgery Clinic, Izmir, Turkey
Published: 04 Jul, 2018
Cite this article as: Üstün M, Karaca AC, Yoldaş T, Sezer TÖ, Çalışkan C. Growing Teratoma Syndrome: A Report of a Rare Case with a detailed Review of the Literature. World J Surg Surgical Res. 2018; 1: 1022.
Abstract
Growing Teratoma Syndrome (GTS) is a rare entity, which requires clinical vigilance for early diagnosis. Surgery remains the sole treatment but it can be complicated especially for the delayed patients. Awareness on the condition can lead physicians to make more accurate diagnosis on the basis of suspicion for the patients with Non-Seminomatous Germ Cell Tumors (NSGCT).
Keywords: Growing teratoma syndrome; Surgery; Nonseminomatous germ cell tumor
Introduction
Growing Teratoma Syndrome (GTS) is a very rare clinical condition that was first described in early 80’s by Logothetis et al. [1]. A detailed review of the disease by Gorbatiy et al. [2] in 2009 reports less than 100 cases since the definition of the condition. It can be generally referred as “benign maturation” of NSGCT, including seminoma and immature teratoma, during or after chemotherapy, with normal serum levels of Alpha-Fetoprotein (AFP) and β- Human Chorionic Gonadotropin (β-HCG), whose metastatic sites consists of well-differentiated teratomatous elements [1]. Complete surgical resection remains the sole treatment of these tumors since they are resistant to radio and chemotherapy [3].
Hereby we represent a typical case of GTS in a 21-year-old individual who was treated with complete surgical resection with a brief review of the literature.
Case Presentation
21-years-old female patient on her 24th gestational week of her first pregnancy presented with abdominal pain and discomfort. Sonographic studies revealed a 12 × 11 centimeters mass arising from left ovary. The measured values of AFP, β-HCG and CA 125 at the time of diagnosis were 1,190, 21 and 87 nano grams per milliliters (ng/ml) respectively. The patient underwent a left oophorectomy and pathologic evaluation of the mass revealed an immature teratoma with serum AFP being 1,416 ng/ml right after the operation.
Pregnancy was later terminated with a Cesarean Section after sufficient maturation of the fetus, with synchronous omentectomy and pelvic paraaortic lymph node dissection. Multiple peritoneal implants were also noted during this procedure. Pathologic evaluation of the specimen confirmed the previous diagnosis of immature teratoma with positive AFP receptors but negative β-HCG and CD-30 receptors. Only one of the dissected 16 lymph nodes was metastatic.
Patient was then administered 4 courses of Bleomycin, Etoposide, and Platinum-cisplatin (BEP) chemotherapy. Radiologic findings revealed progression after chemotherapy but serum levels of AFP was lowered to 53 ng/ml.
Owing to clinical deterioration (growing subhepatic mass complicating respiration, pleural effusion, partial bowel obstruction and severe pain) four courses of second line chemotherapy with paclitaxel, I-phosphamide and cisplatin was administered in hope of clinical answer.
The patient was then referred to Department of General Surgery due to lack of clinical response, compressive complications of the mass and immune and metabolic complications of chemotherapy.
The patient was reevaluated by radiologic imaging that revealed multiple peritoneal implantations with a giant subhepatic mass elevating right diaphragm and shrinking right hepatic lobe and some additional masses on the abdominal wall suggestive of implantation during previous surgeries. Serum AFP was only 3.35 after second line chemotherapy. Positron Emission Tomography (PET) study revealed 2 supraclavicular lymph nodes, suggestive of being metastatic. In addition there were two more lymph nodes in the internal mammary chain also evaluated as suspicious for being metastatic.
A palliative surgery was then performed aiming to relieve obstructive symptoms consisting of subtotal colectomy, partial removal of the subhepatic mass and masses on the abdominal wall and a Brooke’s ileostomy.
The pathological evaluation of this operation specimen revealed no malignant cells but only mature teratoma, which was suggestive of a diagnosis of benign transformation of a previously malignant germ cell tumor.
With the pathological proof, under the guidance of the data from the literature the patient was diagnosed as Growing Teratoma Syndrome and definitive surgery was planned, as the entity was now redefined as a benign condition unresponsive to radio or chemotherapy.
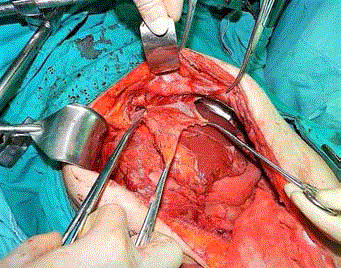
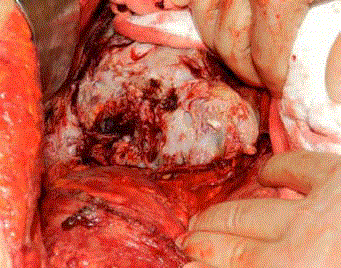
The definitive operation begun with partial peritonectomy of left and right diaphragm (Figure 1) and continued with total removal of all visible peritoneal implants (Figure 2) and the main subhepatic mass (Figure 3). Due to metabolic deterioration of the patient (hypothermia, acidosis and coagulopathy) owing to long operative course (approximately 500 minutes), pelvic peritonectomy was delayed for 24 hours and operation was ended with abdominal packing. After adequate resuscitation of the patient the definitive procedure was completed with the addition of pelvic peritonectomy. Bilateral thorax drains were placed for prevention of massive pleural effusion due to peritonectomy of the diaphragmatic surfaces.
The patient did well for the first postoperative 3 days with major problem being respiratory complications owing to ongoing pleural effusion, which made weaning of the patient from mechanical ventilator impossible. The patient was febrile on the 4th postoperative day and chest x-rays revealed a pneumonic infiltration two days later. The clinical course of the patient was quickly deteriorated right after and she died on the 17th postoperative day due to severe sepsis and multi organ failure.
Figure 1
Figure 2
Figure 3
Discussion
The diagnosis of GTS depends on clinical suspicion that is supported by pathological proof. Clinical suspicion arises from surveillance of serum tumor makers and serial imaging. GTS should be suspected in patients with a history of NSGCT by the presence of, increasing size of metastatic lesions on serial imaging during or after systemic chemotherapy for the treatment of NSGCT and normalized serum tumor markers [1]. All of these criteria were consistent with our patient.
Radiologic progression was observed despite proper chemotherapy but however serum AFP levels were significantly dropped after the administration of chemotherapy and pathologic evaluation after chemotherapy confirmed benign transformation. Earliest reports regarding the benign transformation of germ cell tumors dates were back to 1969 and early 1970’s [4].
However, pathogenesis of the disease remains unclear with some theories attempting to explain this “maturation” progress. The two foremost theories are that: chemotherapy destroys only the immature malignant cells, leaving the mature benign teratomatous elements and chemotherapy alters the cell kinetics toward transformation from a totipotent malignant germ cell toward a benign mature teratoma [5]. A third hypothesis offered by Hong et al. [4] proposes an inherent and spontaneous differentiation of malignant cells into benign teratomas, as suggested by an experimental model.
Reported prevalence of GTS in metastatic NSGCT varies between 1.9% and 7.6% with most recent being a report from MD Anderson Cancer Center, reporting a 2.2% value [1-3]. The most common site for GTS is reported to be retroperitoneum but it can also be observed in the lung, mediastinum, supraclavicular lymph nodes, inguinal lymph nodes, forearm, mesentery and liver [6]. Main mass of our patient was in the liver but there were also pathologic supraclavicular lymph nodes that were revealed wit PET suggestive of bearing disease.
Main pitfall of this rare clinical entity is emphasized to be late diagnosis causing a more extensive surgical dissection with a higher associated risk of adjacent organ injury and increasing the difficulty of the operation [7]. There are some suggestions for avoiding delay, such as regular imaging of every suspected patient after every two cycles of chemotherapy [3]. The diagnosis was also delayed for our patient due to lack of clinical suspicion possibly on the ground that the patient sought medical help in multiple medical centers without proper medical recordings.
There is no effective medical treatment for GTS. There are some experimental attempts of interferon and bevacizumab administration with only relative stabilization of the disease [8,9]. Since the condition is also unresponsive to chemo and radiotherapy the sole treatment of this rare entity is complete surgical resection [1,10].
The 5-year overall survival of GTS is 89%, with mortality related to post-operative complications [2]. Similarly our patient died of severe postoperative complications invoked by extensive surgery.
Conclusion
Although GTS is a benign condition by definition, aggressive clinical behavior can lead to substantial morbidity and mortality. Patients in whom surgery is delayed can develop inoperable disease or be candidates for severe complications.
References
- Logothetis CJ, Samuels ML, Trindade A, Johnson DE. The growing teratoma syndrome. Cancer. 1982; 50(8):1629-35.
- Gorbatiy V, Spiess PE, Pisters LL. The growing teratoma syndrome: Current review of the literature. Indian J Urol. 2009;25(2):186-9.
- Spiess PE, Kassouf W, Brown GA, Kamat AM, Liu P, Gomez JA, et al. Surgical management of growing teratoma syndrome: the M.D. Anderson cancer center experience. J Urol. 2007;177(4):1330-4.
- Hong WK, Wittes RE, Hajdu ST, Cvitkovic E, Whitmore WF, Golbey RB. The evolution of mature teratoma from malignant testicular tumors. Cancer. 1977;40(6):2987-92.
- Carr BI, Gilchrist KW, Carbone PP. The variable transformation in metastases from testicular germ cell tumors: the need for selective biopsy. J Urol. 1981;126(1):52-4.
- Maroto P, Tabernero JM, Villavicencio H, Mesía R, Marcuello E, Solé-Balcells FJ, et al. Growing teratoma syndrome: Experience of a single institution. Eur Urol. 1997;32(3):305-9.
- O'Callaghan AM, Katapodis O, Ellison DW, Theaker JM, Mead GM. The growing teratoma syndrome in a nongerminomatous germ cell tumor of the pineal gland: A case report and review. Cancer. 1997; 80(5):942-7.
- Kattan J, Droz JP, Culine S, Duvillard P, Thiellet A, Peillon C. The growing teratoma syndrome: a woman with nonseminomatous germ cell tumor of the ovary. Gynecol Oncol. 1993;49(3):395-9.
- Mego M, Recková M, Sycova-Mila Z, Obertova J, Brozmanova K, Salek T, et al. Bevacizumab in a growing teratoma syndrome. Case report. Ann Oncol. 2007;18(5):962-3.
- Tangjitgamol S, Manusirivithaya S, Leelahakorn S, Thawaramara T, Suekwatana P, Sheanakul C. The growing teratoma syndrome: a case report and a review of the literature. Int J Gynecol Cancer. 2006; 16(1):384-90.