Short Communication
Clinical Utility of 5-Day-on/2-Day-off UFT/Leucovorin in Postoperative Adjuvant Chemotherapy for Colorectal Cancer
Yukihiko Tokunaga* and Hirokazu Sasaki
Department of Surgery, Kyoto Teishin Hospital, Japan
*Corresponding author: Yukihiko Tokunaga, Department of Surgery, Kyoto Teishin Hospital, Nakagyo-ku, Kyoto, 604-8798, Japan
Published: 04 Jun, 2018
Cite this article as: Tokunaga Y, Sasaki H. Clinical Utility of 5-Day-on/2-Day-off UFT/Leucovorin in Postoperative Adjuvant Chemotherapy for Colorectal Cancer. World J Surg Surgical Res. 2018; 1: 1015.
Abstract
Background: Recent clinical studies proved oral Uracil/Tegafur (UFT)/Leucovorin (LV) regimen, in which the drugs are taken for 28 consecutive days every 35 days, equivalent to an infusional 5-fluorouracil/LV regimen for treatment of Colorectal Cancer (CRC). The 5-day-on/2-day-off schedule for UFT/LV, which is taken for 5 days followed by 2 days off, has been proposed, since the 5-day-on/2-day-off schedule for UFT has been safe and effective. However, clinical utility of 5-day-on/2-day-off schedule for UFT/LV has scarcely been reported in terms of effects and safety.
Methods: We assessed the utility of 5-day-on/2-day-off schedule of UFT/LV in 40 CRC patients, being compared to the consecutive schedule in 20 CRC patients as adjuvant chemotherapy. UFT (300 mg/m2/day)/LV (75 mg/body/day) were taken in both schedules.
Results: In the 5-day-on/2-day-off schedule, 35 of 40 (87%) patients received all scheduled doses of the regimen. The mean relative dose intensity was 0.92. In consecutive schedule, 16 of 20 (80%) patients received all scheduled doses of the regimen. The mean relative dose intensity was 0.87. The toxicity was milder in the 5-day-on/2-day-off schedule than the consecutive schedule. A total of 14 adverse events developed in the 5-day-on/2-day-off schedule, and a total of 29 adverse events developed in the consecutive schedule. Disease-free survival in patients with the 5-day-on/2-day-off schedule was significantly (P=0.05) better than those with consecutive schedule.
Conclusion: The present results indicated that the 5-day-on/2-day-off schedule of UFT/LV would be useful and effective with mild adverse reaction in long-term adjuvant chemotherapy after surgery for CRC.
Keywords: Colorectal cancer; Chemotherapy; UFT/LV; Postoperation
Introduction
Recent clinical studies proved oral Uracil/Tegafur (UFT)/Leucovorin (LV) regimen, in which the drugs are taken for 28 consecutive days every 35 days, equivalent to an infusional 5-Fluorouracil (5-FU)/LV regimen for treatment of Colorectal Cancer (CRC) [1,2]. The UFT/LV regimen has been accepted as one of the adjuvant chemotherapies after surgery. The 5-day-on/2-day-off schedule for UFT/LV, which is taken for 5 week days followed by 2 weekend days off, has been proposed, since the 5-day-on/2-day-off schedule for UFT has been reported to be safe and effective [3-5]. However, clinical utility of 5-day-on/2-day-off schedule for UFT/LV has scarcely been reported in terms of effects and safety. We assessed the utility of the regimen as adjuvant chemotherapy in CRC patients as compared to the consecutive UFT/LV regimen.
Patients and Methods
From January 2006 to June 2010, 60 patients were treated with oral UFT/LV as adjuvant chemotherapy after surgery for CRC. Patients were prospectively allocated to one of the two UFT/LV schedules in two to one ratio of 60 patients, 40 were treated with UFT (300 mg/m2/day)/LV (75 mg/body/day) which is taken for 5 week days followed by 2 weekend days off. One course contained 5 weeks, and 15 courses (75 weeks) were given. The other 20 patients were treated with UFT (300 mg/m2/day)/LV (75 mg/body/day) which is taken for 28 consecutive days every 35 days. One course contained 5 weeks (35 days), and 5 courses (25 weeks) were given. We assessed effects of the regimen in terms of disease-free survival as well as compliance in terms of toxicity, continuity and so on.
Toxicities were graded according to the Common Terminology Criteria for Adverse Events v3.0 (CTCAE) [6]. If hematologic or non-hematologic toxicities of grade 2 or more; UFT/LV was occasionally stopped after consulting with the patients. When hematologic or non-hematologic toxicities of grade 2 developed, the UFT dose was reduced for subsequent course. The patients were free to reject chemotherapy for any reason whatsoever. This study has been approved by institutional review board.
Statistical analyses were undertaken using non-parametric methods including chi-square test, Fisher's exact probability test. Survival curves were calculated using the Kaplan-Meier method, and differences were evaluated with the log-rank test. Probability values from two-tailed test less than 0.05 were considered statistically significant.
Results
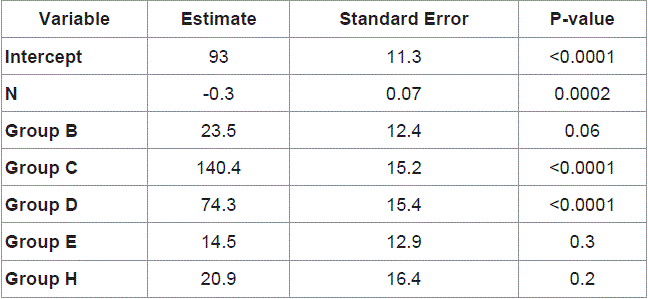
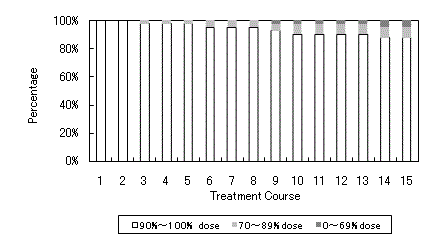
Characteristics of the patients were shown in Table 1. Mean age was 65, ranging from 55 to 79. All patients had a performance status of 0 or 1. Rectal cancer patients accounted for 31.7%. Treatment courses were shown in Table 2. In the 5-day-on/2-day-off schedule, 35 of 40 (87%) patients received all scheduled doses of the regimen. The mean relative dose intensity was 0.92 (Figure 1). Mean treatment courses given were 13.8. In the consecutive schedule, 16 of 20 (80%) patients received all scheduled doses of the regimen. The mean relative dose intensity was 0.87. Mean treatment course given was 4.7.
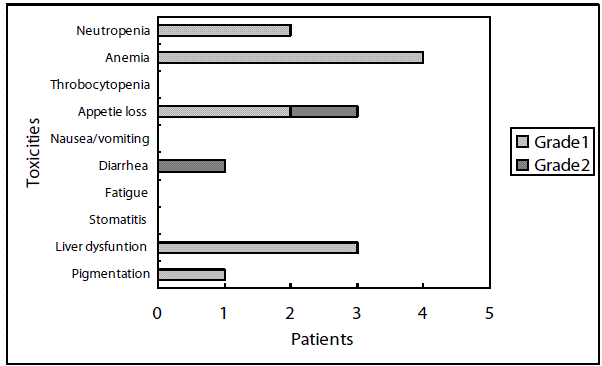
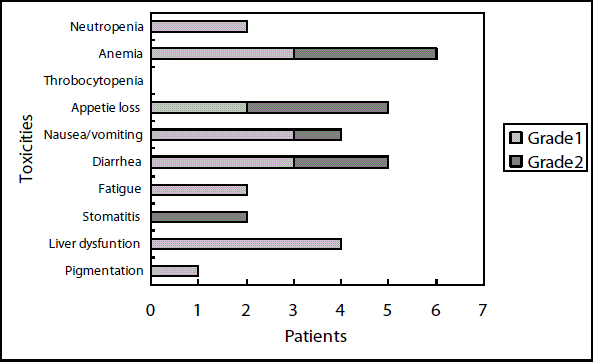
Toxicities were relatively mild and no patients experienced toxicity of grade 3 or 4 in both schedule. A total of 14 patients experienced toxicities in the 5-day-on/2-day-off schedule (Figure 2A), and a total of 29 patients experienced toxicities in the consecutive schedule (Figure 2B). The toxicities were milder in the 5-day-on/2-day-off schedule than the consecutive schedule. There have been 6 cases of discontinuation of the regimens, 5 cases in the 5-day-on/2-day-off schedule and 4 cases in the consecutive schedule. The reasons for discontinuation were recurrence in 2 cases, toxicities in 4 cases, and patient rejection in 3 cases. Disease-free survival in patients with the 5-day-on/2-day-off schedule was significantly (P=0.05) better than those with consecutive schedule (Figure 3).
Table 1
Table 2
Figure 1
Figure 1
Percentage of the patients who actually have taken UFT as compared to scheduled dose of 90% to 100%, 70% to 89%, and 1% to 69% (leucovorin was taken as scheduled).
Figure 2A
Figure 2A
MRI demonstrates a dilated, fluid-filled appendix with thickened, edematous walls and surrounding periappendiceal inflammation, consistent with appendicitis.
Figure 2B
Figure 2b
MRI demonstrates a dilated, fluid-filled appendix with thickened, edematous walls and surrounding periappendiceal inflammation, consistent with appendicitis.
Figure 3
Figure 3
MRI demonstrates a dilated, fluid-filled appendix with thickened, edematous walls and surrounding periappendiceal inflammation, consistent with appendicitis.
Discussion and Conclusion
Recent clinical studies proved oral UFT/LV regimen as conventional regimen, in which the drugs are taken for 28 consecutive days every 35 days, equivalent to an infusional 5-FU/LV regimen for CRC treatment [1,2]. The UFT/LV regimen has been widely accepted as adjuvant chemotherapy in Japan. With conventional consecutive regimen, Carmichael et al. [1] reported that diarrhea developed in 18% and nausea/vomiting in 9%. Douillard et al. [2] reported that non-hematological toxicities were diarrhea (21%), nausea/vomiting (13%).
In NSABP C-06, conventional UFT/LV regimen was used as adjuvant chemotherapy [7]. As the results, grade 3 or more diarrhea developed in 29% and vomiting in 5%. Meguro et al. [8] reported that conventional UFT/LV regimen could be completed in 82.5% patients without any toxicity grade 3 or more. They reported, however, that the most frequent non-hematologic toxicities were fatigue (60%) and appetite loss (50%). Nausea/vomiting and diarrhea developed in 40% and 30% respectively. The present results using the 5-day-on/2-day-off schedule of UFT/LV showed only grade 2 appetite loss (3%) and diarrhea (3%) without any toxicity grade 3 or more.
In the previous chemotherapy using UFT as a single agent with a relatively high dose, the 5-day-on/2-day-off schedule has been reported to provide better results as postoperative adjuvant chemotherapy for CRC than conventional consecutive administration [5]. Furthermore, the 5-day-on/2-day-off schedule has been reported to be safer and to provide better feasibility than conventional consecutive administration even with a relatively high dose [5]. Tegafur is metabolized to 5-FU by the hepatic P-450 drug-metabolizing enzyme. As for 5-FU, two mechanism of anticancer action including concentration-dependent RNA dysfunction and time-dependent DNA synthesis inhibition are known [9]. Antitumor effects are provided when 5-FU concentration is maintained sufficiently for certain duration. In time-dependent DNA synthesis inhibition, adverse effects and toxicities to the normal cells are milder in the intermittent schedule than the consecutive schedule, since cell cycle of cancer cell has been reported to be 3 to 7 days, whereas that of normal cell is 0.5 to 1 day [10,11].
In a preliminary study using UFT/LV, anti-tumor effects of the 5-day-on/2-day-off schedule of UFT/LV were obtained stronger than consecutive regimen, and toxicities were milder in the intermittent schedule than the consecutive schedule [12]. In chemotherapy using UFT/LV, therefore, anti-tumor effects of the 5-day-on/2-day-off schedule of UFT/LV might be obtained stronger than consecutive regimen, since the 5-day-on/2-day-off schedule of UFT/LV could provide a relatively high dose of UFT than consecutive regimen, thanks to milder toxicities [4,5,12].
There have been few studies available to assess the optimal duration of adjuvant chemotherapy for CRC [13,14]. With infusional 5-FU/LV regimens, duration of 6 months has been recommended from the view point of toxicities, cost, and patients convenience, since previous studies showed no significant difference in disease free survival and/or overall survival between the patients treated with adjuvant chemotherapy for 6 months and those for 12 months [13,14]. On the other hand, with oral Tegafur regimens, several clinical studies indicated the efficacy of adjuvant chemotherapy for 1 to 2 years [15,16]. Since recurrence occurred a year after curative resection in about 30% of high-risk patients [17], therefore, it would be useful to evaluate the effects and feasibility of oral UFT/LV in postoperative long-term adjuvant chemotherapy.
The present results indicated that disease-free survival in patients with the 5-day-on/2-day-off schedule was better than those with consecutive schedule with milder adverse reactions. The 5-day-on/2-day-off schedule of UFT/LV would be effective and feasible in long-term adjuvant chemotherapy after surgery for CRC.
References
- Carmichael J, Popiela T, Radstone D, Falk S, Borner M, Oza A, et al. Randomized comparative study of tegafur/uracil and oral leucovorin versus parenteral fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2002;20(17):3617-7.
- Douillard JY, Hoff PM, Skillings JR, Eisenberg P, Davidson N, Harper P, et al. Multicenter phase III study of uracil/tegafur and oral leucovorin versus fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2002;20(17):3605-16.
- Sadahiro S, Mukai M, Tokunaga N, Tajima T, Makuuchi H, Yoshida M, et al. Preliminary study on the optimal dosage schedule for oral tegafur/uracil (UFT) chemotherapy. Int J Clin Oncol. 1998;3(1):7-12.
- Sadahiro S, Suzuki T, Kameya T, Iwase H, Tajima T, Makuuchi H. A pharmacological study of the weekday-on/weekend-off oral UFT schedule in colorectal cancer patients. Cancer Chemother Pharmacol. 2001;47(5):457-60.
- Sadahiro S, Ohki S, Yamaguchi S, Takahashi T, Otani Y, Tsukikawa S, et al. Feasibility of a novel weekday-on/weekend-off oral UFT schedule as postoperative adjuvant chemotherapy for colorectal cancer. UFT compliance study group, kanagawa, japan. Cancer Chemother Pharmacol. 2000;46(3):180-4.
- JCOG/JSCO. Common terminology criteria for adverse events, v3.0 (CTCAE). 2016.
- Lembersky BC, Wieand HS, Petrelli NJ, O'Connell MJ, Colangelo LH, Smith RE, et al. Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: Results of national surgical adjuvant breast and bowel project protocol C-06. J Clin Oncol. 2006;24(13):2059-64.
- Meguro M, Furuhata T, Okita K, Nishidate T, Ishiyama G, Iwayama Y, et al. Clinical compliance with oral uracil/tegafur (UFT) plus leucovorin (LV) regimen as adjuvant chemotherapy in Japanese colorectal cancer patients. Int J Clin Oncol. 2009;14(5):402-7.
- Inaba M, Mitsuhashi Y. Flow cytometric analysis of cell-killing action of 5-fluorouracil in human colorectal cancer cells. Oncol Res. 1994;6(7):303-9.
- Lipkin M, Sherlock P, Bell B. Cell proliferation kinetics in the gastrointestinal tract of man II cell renewal in stomach, ileum, colon and rectum. Gastroenterology. 1963;45:721-9.
- Clarkson B, Ota K, Ohkita T, O’Connor A. Kinetics of proliferation of cancer cell in neoplastic effusions in man. Cancer. 1965;18(10):1189-213.
- Sadahiro S, Suzuki T, Ishikawa K, Nakamura T, Saguchi T, Kamijo A, et al. Preliminary study of the optimal dosing schedule for oral UFT/leucovorin chemotherapy. Anticancer Res. 2004;24(2B):625-30.
- O’Connell MJ, Laurie JA, Kahn M, Fitzgibbons RJ, Erlichman C, Shepherd L, et al. Prospective randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol. 1998;16(1):295-300.
- Haller DG, Catalano PJ, Macdonald JS, O'Rourke MA, Frontiera MS, Jackson DV, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: Final report of Intergroup 0089. J Clin Oncol. 2005;23(34):8671-8.
- Kato T, Ohashi Y, Nakazato H, Koike A, Saji S, Suzuki H, et al. Efficacy of oral UFT as adjuvant chemotherapy to curative resection of colorectal cancer: Multicenter prospective randomized trial. Langenbecks Arch Surg. 2002;386(8):575-81.
- Sakamoto J, Ohashi Y, Hamada C, Buyse M, Burzykowski T, Piedbois P. Efficacy of oral adjuvant therapy after resection of colorectal cancer: 5-year results from three randomized trials. J Clin Oncol. 2004;22(3):484-92.
- Sadahiro S, Suzuki T, Ishikawa K, Nakamura T, Tanaka Y, Masuda T, et al. Recurrence patterns after curative resection of colorectal cancer in patients followed for a minimum of ten years. Hepatogastroenterology. 2003;50(53):1362-6.