Case Report
Immature Gastric Teratoma Arising from Lesser Curvature
Dinesh Kumar Barolia1, Vinay Mathur1, Pradeep Kumar Gupta1, Dileep Garg1, Raj Kumar Yadav2, Neelam Dogra3 and Praveen Mathur1*
1Department of Pediatric Surgery, SMS Medical College, India
2Department of Radiology, SMS Medical College, India
3Department of Anesthesiology, SMS Medical College, India
*Corresponding author: Praveen Mathur, Department of Pediatric surgery, Sir Padampat Mother and Child Health Institute, SMS Medical College, Jaipur, Rajasthan, India
Published: 29 May, 2018
Cite this article as: Barolia DK, Mathur V, Gupta PK, Garg D, Yadav RK, Dogra N, et al. Immature Gastric Teratoma Arising from Lesser Curvature. World J Surg Surgical Res. 2018; 1: 1013.
Abstract
Gastric teratoma is a rare tumor in neonate. The incidence of gastric teratoma is less than 1% of all types of teratoma. While it arises from greater curvature of stomach, it may be exogastric or endogastric variety. Most gastric teratoma is mature. There are only 13 reported cases of immature gastric teratoma arising from lesser curvature of stomach. We are reporting such a case with review of literature.
Keywords: Immature gastric teratoma; Stomach; Teratoma; Lesser curvature
Introduction
Germ cell tumors are grouped as gonadal and extra gonadal. Gastric teratoma is an extra gonadal variant of germ cell tumor. These tumors derived from three embryonic layers, viz. ectoderm, and mesoderm, endoderm which contains various types of epithelium, bony tissue, cartilaginous tissue, muscle tissue, adnexal tissue and glial tissue. Gastric teratoma is a rare entity having incidence less than 1% in all type of teratoma [1]. Most common site of origin of gastric teratoma is greater curvature and posterior wall [1,2]. With incidence of exogastric teratoma, 58% to 70% and endogastric teratoma 30% [3], it is more common in males than females. It presents with lump abdomen, vomiting, respiratory distress, upper gastro-intestinal bleeding [4].
Case Presentation
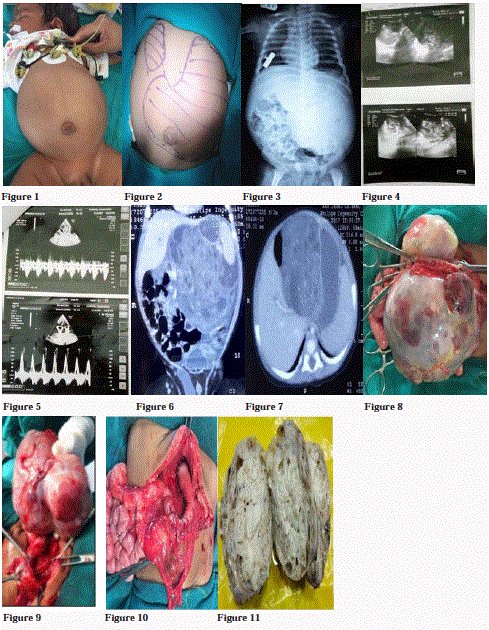
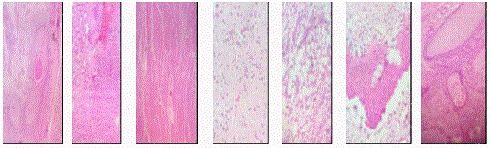
A three month old male child, weighing 6 kg, born full term, normal vaginal delivery to Gravida 2 Para 2 (G2P2) mother was admitted with chief complaint of non-bilious vomiting since two weeks and progressive abdominal distension since one month (Figure 1). On examination, the baby was dehydrated and morose. There were two palpable lumps, one in right hypochondrium and another one in left hypochondrium, which was extending up to umbilical region (Figure 2). Margin of the lump was well-defined and firm in consistency with smooth surface. Routine blood investigations were within the normal limits. The tumor Alpha Fetoprotein (AFP) marker was more than 1,000 international units with X-ray flat plate abdomen and chest showing a large soft tissue mass shadow. While it was found to occupy left side of abdomen with displacement of the bowel loops towards right side, it also showed pear shaped heart shadow (Figure 3). Ultrasonography showed mixed echogenic solid cystic mass of 11 cm × 8 cm × 12.2 cm with internal calcification, arising from pancreas suggesting pancreatic-blastoma (Figure 4). The patient was diagnosed to have cyanotic heart disease. 2D echo showed patent ductus arteriosus with enlarged left atrium and ventricle (Figure 5). The CT scan showed 9.5 cm × 8.1 cm × 11.2 cm lesion with multiple foci of calcification noted, involving body and tail of pancreas extended in lesser sac with the mass shifted the bowel loops to the right side (Figures 6 and 7). Exploration revealed dumb-belled shaped solid-cystic mass arising from lesser curvature of stomach exclusively (Figures 8 and 9). Pancreas was not found to be involved contrary to ultrasonography and CT finding. Complete excision of the mass was done and defect was repaired in two layers (Figure 10). Gross cut section of mass showed solid cystic area filled with mucinous material (Figure 11). On microscopic examination, cyst wall was lined with stratified squamous epithelium, intestinal epithelium and respiratory tract epithelium. Solid part showed muscle tissue, glial tissue, bony tissue, and adipose tissue, bony and cartilaginous tissue (Figure 12). Overall histopathological findings suggest it to be grade 3, immature gastric teratoma. No chemotherapy was given to patient and the post-operative recovery was uneventful. There was no recurrence of tumor on follow-up.
Discussion
Teratoma is relatively common embryonic neoplasms arising from totipotent cells and contains elements from all the three germ layers [5]. In infancy and early childhood, the most common site of teratomas is the extra gonadal region, which includes sacrococcygeal, mediastinal; presacral rarely occurs in retroperitoneal, cervical and intra cranial region [6]. Whereas after childhood; they are most commonly located in the gonads. Majority of gastric teratoma (90%) occurs in neonatal period and infancy with males largely affected [7]. Typical presenting symptoms of gastric teratomas are lump abdomen, abdominal distension, vomiting, haematemesis (commonly with endogastric variety) and respiratory distress (commonly with exogastric variety) [4]. Gastric teratoma most commonly arises from the greater curvature and posterior wall of stomach and lesser curvature is an uncommon site [1,2]. Whereas it is common in neonates, Eusterman and sentry in 1922 reported the first case of gastric teratoma in 31 year old male [8].
Most of the gastric teratomas are mature in nature with immature gastric teratoma being a rare tumor. Mature and immature gastric teratoma differentiated on the basis of glial tissue and other derivatives of germ cell layer. Mature glial tissue and other derivatives of at least one germ layer present in mature gastric teratoma. Immature gastric teratoma contains immature glial tissue along with other derivatives of germ cell layer and is malignant potential [9]. Immature gastric teratoma is graded 1,2,3 by the amount of foetal tissue and on the basis of mitotic activity in cells. Grade 1 contains immature tissue at one site in whole slide whereas grade 2 contains immature tissue at <4 sites and grade 3 contains at >4 sites in a slide [10,11]. Radiological findings are solid-cystic mass with internal calcification [12]. The AFP level is very helpful to know the recurrence and after complete excision of gastric teratoma, level of AFP decreases. We are adding one case to the already reported 13 cases of immature gastric teratoma arising from lesser curvature (Table 1). Four cases were diagnosed in neonatal age [13-16] and one case was diagnosed in antenatal period [16]. Rest of the cases were diagnosed between 3 to 8 months [1,4,16,18-22]. Immature gastric teratoma also has a predilection for male sex. Four reported cases had AFP level within normal limits and seven had a raised level.
Table 1
Figure 1-Figure 11
Figure 1-Figure 11
Figure 1: Marked abdominal distension.
Figure 2: Surface marking of lump abdomen, showing two lumps. Left one is extending up to umbilicus.
Figure 3: X-ray flat plat abdomen and chest showed soft tissue radiodensity in left side of abdomen with displacement of bowel loops towards right side. Pear
shaped heart shadow seen and also fullness in aorto-pulmonary window.
Figure 4: USG showed mixed echogenic mass with cystic changes and small specks of calcification, not separable from pancreas and lesser curvature of stomach.
Figure 5: Trans thoracic echo cardiography showed continuous flow at origin of left pulmonary arch on continuous wave Doppler imaging, suggestive of patent
ductus arteriosus.
Figures 6 and 7: Coronal and axial CT images showed solid enhancing mass with cystic changes and specks of calcification, with small bowel loops shifted
towards right side.
Figure 8 and 9: Anterior and posterior view of Dumb-bell shaped lump abdomen arising from lesser curvature of stomach.
Figure 10: After complete excision of mass with defect repaired.
Figure 11: Gross cut section of mass showed solid cystic component.
Figure 12
Figure 12
Haematoxylin Eosin (HE) stained micro photograph (100X) showing derivatives of all three germ layers, like squamous epithelium, intestinal epithelium,
muscle tissue, glial tissue, adipose tissue, bony tissue and cartilaginous tissue in sequential order.
Conclusion
Immature gastric teratoma is an extremely rare tumor. It is common in male. Presenting symptom is abdominal distension. Lesser curvature is a least common site of origin. Complete surgical excision with long term follow up with monitoring of AFP level is the best protocol for treatment plan.
Acknowledgement
The authors would like to express their deep gratitude to Dr. Shubha Gupta and Dr. Mansi Faujdar, Honorary Consultants, SDMH, Jaipur for providing valuable information about histopathology. PM acknowledge Dr. Prashanth Suravajhala, Research Scientist, Birla Institute of Scientific Research, Jaipur, India for his help on the manuscript.
References
- Gupta DK, Srinivas M, Dave S, Agarwala S, Bajpai M, Mitra DK. Gastric teratoma in children. Pediatr Surg Int. 2000;16(5-6):329-32.
- Avanoglu A, Ulman I, Balik E. Case report of gastric teratoma in a female infant. J Pediatr Surg. 1996;31(2):327.
- Gangopadhyay AN, Pandit SK, Sinha A. Gastric teratoma–review of literature. Indian J Pediatr. 1992;59:541-4.
- Çorapçioglu F, Ekingen G, Sarper N, Guvenc BH. Immature gastric teratoma of childhood: A case report and review of the literature. J Pediatr Gastroenterol Nutr. 2004;39(3):292-4.
- Gamanagatti S, Kandpal H. Gastric teratoma. Singapore Med J. 2007;48(4):e99-101.
- Singh M, Rattan KN, Kadian YS, Hasija N. Gastric teratoma: A rare neoplasm. J Neonatal Surg. 2012;1(2):28.
- Gobel U, Calaminus G, Engert J, Kaatsch P, Gadner H, Bökkerink JP, et al. Teratomas in infancy and childhood. Med Pediatr Oncol. 1998;31(1):8-15.
- Eusterman GB, Sentry EG. Benign tumors of the stomach: Report of twenty seven cases. Surg Gynecol Obstet. 1922;34:372-8.
- Bourke CJ, Mackay AJ, Payton D. Malignant gastric teratoma: case report. Pediatr Surg Int. 1997; 12(2-3):192-3.
- Sharma A, Arora R, Gupta R, Dinda AK. Immature gastric teratoma in an infant: report of a case and review of the literature. Indian J Pathol Microbiol. 2010;53(4):868-70.
- Norris HJ, Zirkin HJ, Benson WL. Immature (malignant) teratoma of the ovary : a clinical and pathologic study of 58 cases. Cancer. 1976;37(5):2359-72.
- Bowen B, Ros PR, McCarthy MJ, Olmsted WW, Hjermstad BM. Gastrointestinal teratomas: CT and US appearance with pathologic correlation. Radiology. 1987;162(2):431-3.
- Kim MC, Park CM, Lee JH, Kim DC. Immature Gastric Teratoma in a Neonate. J Korean Gastric Cancer Assoc. 2003;3(3):158-60.
- Ukiyama E, Endo M, Yoshida F, Tezuka T, Kudo K, Sato S, et al. Recurrent yolk sac tumor following resection of a neonatal immature gastric teratoma. Pediatr Surg Int. 2005;21(7):585-8.
- Ahmad R, Gupta R, Mathur P. Immature gastric teratoma originating from lesser curvature: first reported case in neonates. Rajasthan Med J. 2010;09-10:51-3.
- Aihole JS, Babu MN, Jadhav V, Javaregowda D. Gastric teratoma: An unusual presentation and location. Indian J Med Paediatr Oncol. 2017;38(4):563-5.
- Herman TE, Siegel MJ. Congenital gastric teratoma. J Perinatol. 2008;28:786-7.
- Moriuchi A, Nakayama I, Muta H, Taira Y, Takahara O. Gastric teratoma of children: a case report and review of the literature. Acta Pathol Jpn. 1977;27(5):749-58.
- Utsch B, Fleischhack G, Knöpfle G, Hasan C, Bode U. Immature gastric teratoma of the lesser curvature in a male infant. J Pediatr Gastroenterol Nutr. 2001;32(2):204-6.
- Valenzuela-Ramos MC, Mendizabal-Mendez AL, Rıos-Contreras CA, Rodrıguez-Montes CE. Pediatric gastric teratoma. J Radiol Case Rep. 2010;4(10):6-13.
- Singh S, Rawat J, Ahmed I. Immature extragastric teratoma of infancy: a rare tumour with review of the literature. BMJ Case Rep. 2011;2011.
- Junhasavasdikul T, Ruangwattanapaisarn N, Molagool S, Lertudomphonwanit C, Sirachainan N, Larbcharoensub N. Immature gastric teratoma in an infant: a case report and review of the literatures. Clin Case Rep. 2016;4(10):962-967.