Research Article
Evaluation of the Factors Affecting the Patency of Arteriovenous Fistulas used for Hemodialysis in End Stage Renal Failure Patients
Eyupserhat Calik1*, Umit Arslan1, Ednan Bayram2 and Bilgehan Erkut1
1Department of Cardiovascular Surgery, Atatürk University Medical Faculty, Erzurum, Turkey
2Department of Cardiology, Atatürk University Medical Faculty, Erzurum, Turkey
*Corresponding author: Bilgehan Erkut, Atatürk University Medical Faculty, Department of Cardiovascular Surgery, Erzurum, Turkey
Published: 29 May, 2018
Cite this article as: Erkut B, Calik E, Arslan U. Evaluation of the Factors Affecting the Patency of Arteriovenous Fistulas used for Hemodialysis in End Stage Renal Failure Patients. World J Surg Surgical Res. 2018; 1: 1011.
Abstract
The continuity of vascular access still causes major problems and cost loss in end stage renal failure patients. Many factors may be effective in providing primary arteriovenous fistula patency. We examined the effects of various factors on fistulas in 821 chronic renal insufficiency patients. From 2010 to 2018, 821 arteriovenous fistulas were created by the 2 different cardiovascular surgery clinics (University and Training-Research Hospital) for hemodialysis. The average age of the patients is 57 years (9 to 71 years). Primer patency was described as the time until the patient needed a second surgical procedure. None of the patients had pathology requiring additional surgery. Age, sex, smoking habits, hypertension, diabetes mellitus, malignant history, fistula localization and previous dialysis catheter placement were analyzed.
There was no statistically significant difference between the patency rates of the between the upper and lower arm arteriovenous fistulas formed in fistula-created patients. Diabetes mellitus (p=0.0001), hypertension (p<0.0005), smoking habits (p<0.0005), previous catheter placement (p<0.0007) and age (p=0.0008) were found to be factors affecting the primary patency of arteriovenous fistulas. And our results showed that these risk factors affect the fistula patency, negatively.
Keywords: Arteriovenous fistula; Hemodialysis; Fistula patency
Introduction
As the number of patients requiring hemodialysis increases, the number of patients requiring multiple repairs due to unsuccessful attempts is steadily increasing. A large number of patients who need dialysis cannot become kidney transplant candidates [1]. The average number of hospital days per year for hemodialysis patients is 13.8. Complications due to vascular interventions and vascular disruptions due to recurrent interventions are the major cause of patient-related morbidity [2,3]. For hemodialysis patients, any vascular intervention is of limited duration and each patient has a limited number of access sites for vascular intervention. In 1966 Brescia et al. [4] provided groundbreaking applications and this arteriovenous fistula-like shape created a great convenience in terms of hemodialysis patients. Since then, it has begun to be used in almost all hemodialysis patients and is still being used as the first choice [4].
By describing possible factors affecting the long-term patency of the fistula, we can assume that the problems associated with the fistula that may occur later may be delayed. The purpose of this study was to evaluate whether there are any prognostic associations of vascular access parameters of various factors in patients with chronic renal failure.
Methods
821 arteriovenous fistulas were created in the clinics of the two hospitals (Ataturk University and Training and Research Hospital) in cardiovascular surgery. 499 (61%) were male and 322 (39%) were female. Their mean age was 57 years (range 9 to 71 years). The patients prospectively underwent follow-up for 5 years, and retrospectively to the day of the fistula creation. Patients were followed up until the day of the kidney transplant or death. Among our patients, glomerulonephritis (41.7%), chronic pyelonephritis (19.2%), polycystic kidney disease and diabetes mellitus were the most frequent causes of end-stage renal disease and hemodialysis indications. Patient's age, gender, presence of smoking habits, hypertension, diabetes mellitus, malignant history, previous dialysis catheterization, and fistula localization were recorded. Arteriovenous fistulas were created under local anesthesia by two of the senior staff surgeons of cardiovascular surgery. The non-dominant upper extremity, usually the left, was preferentially used. The lower extremity was avoided because of concern for infection, effect of peripheral vascular disease and particularly, patient inconvenience. Hemodialysis through the new blood access was begun no sooner than 3 weeks after creation of the arteriovenous. Generally, arteriovenous fistulas should be performed from distal to proximal, using the patient’s own otogenic veins for hemodialysis. Distal arteriovenous fistulas are preferred in order to enlarge the puncture area. The most common types of surgical intervention to create an arteriovenous fistula were snuff-box, Brescia-Cimino, basilic vein transposition, brachiocephalic, upper radio cephalic. We generally used end-to-side anastomosis technique. Anastomosis was constructed with a continuous 6/0 or 7/0 prolene suture. Special care was taken to ensure the formation of a smooth hemi circle for the divided vein; the wound was then closed in a single layer and the hand was kept elevated, coupled with active finger exercises for 24h to 48h. The patients prospectively underwent follow-up for 3.4 ± 1.2 years (mean 3.1 years). If the patient underwent kidney transplantation or died during the follow-up period, the follow-up ended at the day of transplantation or day of death. The patients with early fistula failure were accepted.
Statistical Analysis
Gender, diabetes mellitus, hypertension, smoking habit, malignant history and previous dialysis catheter placement were compared with chi square test and fisher's exact test while student t-test was used to compare ages between the patency and non-patency groups. Statistically, 0.05 levels were accepted as significant. Kaplan Meier survival analysis and log rank test were used to assess the obstruction status of the fistula for 5 years. Cox regression analysis was used to determine whether the factors had effectiveness on non-patency time. Non-patency time was considered as the “dependent variable” for analysis, whereas sex, the existence of hypertension and diabetes mellitus, malignant history, smoking habit, fistula localization, and previous dialysis catheter placement were considered as “non-dependent variables”. Age-related data were presented with the mean ± standard deviation of the arithmetic mean. Categorical variables were expressed as counts and percentages, and the results of the cox regression analyzes were evaluated as Odds Ratio (OR) and 95% Confidence Interval (CI).
Results
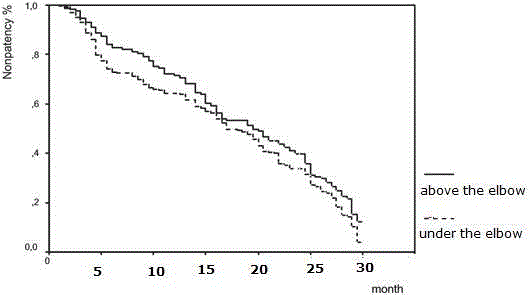
The analysis included 821 patients; 499 (61%) men and 322 (39%) women with median age of 57 (range 9 to 71). The mean ages were 42.1 ± 9.6 and 40.7 ± 10.5 for the patency and non-patency group, respectively, and there was no statistical significance (t=0.88 ± df: 388, p=0.522). The overall primary patency rates were 85.2%, 72.1%, 52.8%, 42.9% and 18.2% after 1, 2, 3, 4, and 5 years, respectively, in under-below fistulas. In upper-below (upper brachial fistulas) these rates were 88.0%, 76.2%, 59.8%, 51.9% and 22.1% after 1, 2, 3, 4, and 5 years, respectively (Figure 1). There was no significant difference between the upper-arm fistulas and the lower-arm fistulas for the time of non-patency (Log rank p=0.101). The mean closure time of the under the elbow fistula was 28.3 months; the above the elbow fistula was 29.4 months. Glomerulonephritis and chronic pyelonephritis were determined to be the most frequent causes of hemodialysis (41.7% and 19.2%).
Comparisons of patency and non-patency fistulas with regard to some variables are presented in Table 1. The patency rates were not influenced by sex (p=0.899), fistula localization (p=0.222), and malignant history (fisher exact test, p=0.501). Diabetes mellitus was found in 96 (11.6%) patients, which affected the fistula patency. In both fistula types, arteriovenous patency was significantly induced in diabetic patients (p=0.021). Hypertension was detected in 106 patients (12.9%). In these patients, the patency of arteriovenous fistulas was worse than in other patients (fisher exact test, p=0.018). In 662 (80.6%) patients, the arteriovenous fistula created had been inserted in the hemodialysis catheter with the subclavian or jugular vein in the same extremity, previously. In these patients, arteriovenous fistula patency was significantly reduced (p=0.009). 512 of our patients (62.3%) were active smokers. In these patients, arteriovenous fistulas were worse than non-smoking patients (fisher exact test, p=0.042). 422 of our patients were over 60 years of age. When these patients were compared with patients below 60 years of age, patency rates are lower in patients over 60 years of age (student t-test, p=0.012).
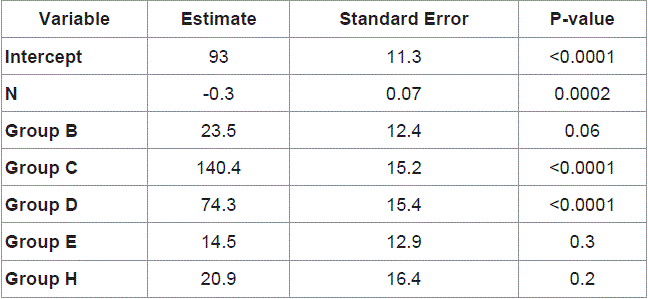
The factors which effect non-patency in the 5 year follow-up were evaluated using cox regression analysis. According to the analysis, the existence of diabetes mellitus (OR: 1.99, 95% CI: 1.42-2.79), smoking (OR: 0.522, 95% CI: 0.392-0.788), hypertension (OR: 3.122, 95% CI: 2.11-5.09), previous catheter placement (OR: 2.47, 95% CI: 1.69-3.71), and age (OR: 0.633, 95% CI: 0.209-1.110) were found to be the factors increasing non-patency (Table 2).
Table 1
Table 2
Table 2
Cox Regression analysis results of the factors affecting arteriovenous fistula patency, negatively.
Figure 1
Figure 1
Results between the upper-arm fistulas and the lower-arm fistulas for the time of non-patency.
Discussion
The prevalence of arteriovenous fistula in our patients ranged from 14 to 64 months (median 32). This is similar to the reports presented by various authors [5,6], which is inadequate according to the other results [7].
The creation of an arteriovenous fistula is an important activity that requires discipline for the quality of the patient’s future lives. In some countries, a coordinated study has been conducted to establish the most successful arteriovenous fistula. In these countries, arteriovenous fistula is a professional surgical procedure that nephrologists, radiologists and surgeons organize and evaluate together [6-8]. The creation of fistulas should be given to a limited number of special surgeons because good results are only provided by surgeons who require specialization. All arteriovenous fistulas were created by senior surgeons in this study.
Distal arteriovenous fistula is still the gold-standard access for hemodialysis. If these are not possible, middle-arm and proximal-arm arteriovenous fistulas should be always investigated before committing to proximal procedures. The upper arm fistulas patencies were significantly longer in the various studies than in the lower-arm [8]. In our study, we found patency rates at 85.2%, 72.1%, 52.8%, 42.9% and 18.2% after 1, 2, 3, 4, and 5 years, respectively, in under-below fistulas. In above-below (upper brachial fistulas) these rates were 88.0%, 76.2%, 59.8%, 51.9% and 22.1% after 1, 2, 3, 4, and 5 years. But, there was no significant difference between the upper-arm fistulas and the lower-arm fistulas for the time of non-patency, which is consistent with some studies [8,9] and opposite to others [10,11].
The most commonly used anastomosis technique to create an arteriovenous fistula is the end-to-side technique. While, prior, the side-to-side and end-to-end anastomosis technique are performed, these techniques have mostly been abandoned and end-to-side technique become the most frequently used technique [4]. Although all three techniques had advantages and disadvantages, we used the end-to-side technique in all patients. In this series and others [12,13], an end-to-side (vein-to-artery) anastomosis has been used exclusively to minimize the risk of digital ischaemia, venous hypertension, or edema, which can occur with end-to-end and side-to-side fistulas [14,15].
The primary purpose was to create a forearm fistula. This strategy may have led to the need for additional revisions, but it may save future access sites [16,17]. In general, fistulas created for hemodialysis should be made from the patient's autogenous veins and distal to proximal (snuff-box, Brescia-Cimino, upper radiocephalic, brachiocephalic). Distal arteriovenous fistulas are preferred in order to enlarge the puncture area. For example, the snuff-box arteriovenous fistula has the following advantages: a) It is the most distal site for the arteriovenous fistula and therefore gives a long segment of vein for needling; the close proximity between the radial artery and the cephalic vein in the anatomical snuff-box allows easy anastomosis without mobilization and transmonest factor limiting the duration of fistula function and position of the vein; b) Due to the smaller caliber of the artery, the risk of the bleeding site was under-run, but the fistula developing a steal phenomenon or cardiac failure subsequently thrombosed. Although there was no difference between proximal and distal fistulas in terms of statistical patency, we preferred creating from distal to proximal in order to enlarge the puncture area in the arteriovenous fistula [18,19].
There was no statistical difference in terms of gender in our patients. There was also no difference between the genders related to fistula patency. This result is consistent with the survey of some author [20-22], but opposite to the study of the other author [23]. As reported by eggers, an ever-increasing proportion of patients aged >60 were also recorded in our study [24]. In addition, most of our patients seem to be active smoking stories. Our results showed that the survival of arteriovenous fistula was age-related and active smoker, which is consistent with some [25-27], and opposite to other reports [21,23].
Many studies have investigated the effect of localization of arteriovenous fistula on fistula patency. Some of the authors have been found to be ineffective in terms of patency [11,28]. As opposed to this, some authors have argued that the patency rates of arteriovenous fistulas above the elbow are higher than those under the elbow [29]. In our study, no difference was found between the patency rates of the arteriovenous fistulas over the elbow.
Hypertension is one of the most important factors that can cause vascular injury. Negative effects of hypertension on endothelial injury have been shown in the studies. The adverse effects on the arteriovenous fistula in hemodialysis patients have been proved in many studies [27-29]. However, in some studies, it was also said that it was negligible to affect the fistula patency [12]. It has been shown statistically that hypertension in our series affects the patency of the arteriovenous fistula, negatively.
Gibson et al. [17] described an increased risk of revision in diabetic patients, a finding in line with our own observation. Diabetes mellitus may influence the formation of intimal hyperplasia at the anastomosis or venous valve. Manne et al. [28] published an article that suggests that diabetes does not affect fistula patency [29]. In our series, diabetes mellitus was found in 49 (11.9%) of patients. This had an effect on the fistula patency. In both fistula types, arteriovenous fistula patency was significantly reduced in diabetic patients.
Coagulation disorders are common in cancer patients. Hemostatic abnormalities are present in a majority of patients with cancer. These patients may have increased platelet aggregation, abnormal activation of coagulation cascade, release of plasminogen activator, and/or decreased hepatic synthesis of anticoagulant proteins like protein C and antithrombin III. These abnormalities, however, are unable to predict subsequent development of thromboembolic or hemorrhagic complications [22,30]. Malignant neoplasm was detected in 38 patients. In patients with malignant neoplasm, blood coagulation impairment may have been the main cause of the shorter patency of arteriovenous fistula. But we could not detect any difference in terms of arteriovenous fistula patency when comparing patients with cancer to other patients.
Occlusion in an arteriovenous fistula may be due to a technical defect in the formation of the fistula, inadequate vascular diameters, or proximal obstruction in the venous system [14,31]. It has also been suggested that fistula formation in patients who have started hemodialysis using temporary access is associated with a higher incidence of fistula failure [32]. Subclavian venous occlusion may cause fistula failure. Subclavian venous stenosis or occlusion can be seen in 50% of patients who have previously undergone hemodialysis catheterization [32]. 662 (81%) of our patients with created arteriovenous fistulas had the hemodialysis catheter inserted in the subclavian or jugular vein in the same extremity, previously. In these patients, arteriovenous fistula patency was significantly reduced. It is important to instruct the patient in time and to motivate him so that forearm veins are preserved. Puncture of a vein will leave a scar. When a fistula is created, such scars interfere with harmonious dilation and remodeling, cause turbulent flow, and predisposition to stenosis. The veins of both arms, not only of the dominant arm, should remain untouched. For vein puncture, the veins of the dorsum of the hand should be used as an alternative. In addition, previous catheter placement in the same arm may affect fistula patency. Due to catheter placement, the vein may be thickened, and may cause fistula failure. Thus, before fistula creation, veins of the arm must not be used, if possible, for catheter procedure [18].
Conclusion
Finally, our data analysis showed that some risk factors, such as age, diabetes mellitus, smoking history, hypertension, previous catheter placement, and decreased patency. Although there was no significant difference between the upper-arm fistulas and the lower-arm fistulas for time of non-patency in our study, in order to enlarge the puncture area for later surgical interventions, arteriovenous fistulas should be performed from distal to proximal with the patient’s own otogenic veins used for hemodialysis. These early findings need to be confirmed in larger and longer follow-up studies.
References
- Jones KR. Factors associated with hospitalization in a sample of chronic hemodialysis patients. Health Serv Res. 1991;26(5):671-99.
- Rocco MV, Bleyer AJ, Burkart JM. Utilization of inpatient and outpatient resources for the management of hemodialysis access complications. Am J Kidney Dis. 1996;28(2):250-6.
- Chazan JA, London MR, Pono L. The impact of diagnosis related groups on the cost of hospitalization for end-stage renal disease patients at Rhode Island hospital from 1987 to 1990. Am J Kidney Dis. 1992;19(6):523-5.
- Brescia MJ, Cimino JE, Appel K, Hurwich BJ. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N Engl J Med. 1966;275:1089-92.
- Burger H, Kluchert BA, Kootstra G, Kitslaar PJ, Ubbink DT. Survival of arteriovenous fistulas and shunt for hemodialysis. Eur J Surg. 1995;161(5):327-34.
- Zibari GB, Rohr MS, Landreneau MD, Bridges RM, DeVault GA, Petty FH, et al. Complications from permanent hemodialysis vascular access. Surgery. 1988;104(4):681-6.
- Chazan JA, London MR, Pono LM. Long-term survival of vascular accesses in a large chronic hemodialysis population. Nephron. 1995;69(3):228-33.
- Dixon BS, Novak L, Fangman J. Hemodialysis vascular access survival: Upper-arm native arteriovenous fistula. Am J Kidney Dis. 2002;39(1):92-101.
- Bartova V, Vanecek V, Valek A. Snuffbox fistula - beter vascular access for hemodialysis. Dialysis & Transplantation. 1984;13:631-32.
- Polo JR, Romero A. Brachiocephalic fistulas for vascular access. Nephron. 1989;52:105-6.
- Tautenhahn J, Heinrich P, Meyer F. Arteriovenous fistulas for hemodialysis-patency rates and complications-a retrospective study. Zentralbl Chir. 1994;119(7):506-10.
- Astor BC, Coresh J, Powe NR, Eustace JA, Klag MJ. Relation between gender and vascular access complications in hemodialysis patients. Am J Kidney Dis. 2000;36(6):1126-34.
- Sekar N. Snuff-box arteriovenous fistulas. Int Surg. 1993;78(3):250-51.
- Harder F, Landman J. Trends in access surgery for hemodialysis. Surgery Annual. 1984;16:135-49.
- Marx AB, Landman J, Harder FH. Surgery for vascular access. Curr Probl Surg. 1990;18(1):15-46.
- Glickman MH, Stokes GK, Ross JR, Schuman ED, Sternbergh WC, Lindberg JS, et al. Multicenter evaluation of a polyurethanurea vascular access graft as compared with the expanded poly tetrafluoro ethylene vascular access graft in hemodialysis applications. J Vasc Surg. 2001;34(3):465-73.
- Gibson KD, Gillen DL, Caps MT, Kohler TR, Sherrard DJ, Stehman-Breen CO. Vascular access survival and incidence of revisions: A comparison of prosthetic grafts, simple autogenous fistulas, and venous transposition fistulas from the United States renal data system dialysis morbidity and mortality study. J Vasc Surg. 2001;34(4):694-700.
- Erkut B, Unlü Y, Ceviz M, Becit N, Ateş A, Colak A, et al. Primary arteriovenous fistulas in the forearm for hemodialysis: Effect of miscellaneous factors in fistula patency. Ren Fail. 2006;28(4):275-81.
- Ates A, Ozyazicioglu A, Yekeler I, Ceviz M, Erkut B, Karapolat S, et al. Primary and secondary patency rates and complications of upper extremity arteriovenous fistulae created for hemodialysis. Tohoku J Exp Med. 2006;210(2):91-7.
- Culp K, Flanigan M, Taylor L, Rothstein M. Vascular access thrombosis in new hemodialysis patients. Am J Kidney Dis. 1995;26(2):341-46.
- Puskar D, Pasini J, Savic I, Bedalov G, Sonicki Z. Survival of primary arteriovenous fistula in 463 patients on chronic hemodialysis. Croation Medical Journal. 2002;43(3):306-11.
- Goad KE, Gralnick HR. Coagulation disorders in cancer. Hematol Oncol Clin North Am. 1996;10(2):457-84.
- Benaragama KS, Barwell J, Lord C, John BJ, Babber A, Sandoval S. Post-operative arterio-venous fistula blood flow influences primary and secondary patency following access surgery. J Ren Care. 2018.
- Eggers PW. Effect of transplantation on the Medicare end-stage renal disease program. N Engl J Med. 1988;318:223-9.
- Goldwasser P, Avram MM, Collier JT, Michel MA, Gusik SA, Mittman N. Correlates of vascular access occlusion in hemodialysis. Am J Kidney Dis. 1994;24(5):785-94.
- Lazarides MK, Iatrou CE, Karanikas ID, Kaperonis NM, Petras DI, Zirogiannis PN. Factors affecting the lifespan of autologvous and synthetic arteriovenous access routes for hemodialysis. Eur J Surg. 1996;162(4):297-301.
- Green KB, Silverstein RL. Hypercoagulability in cancer. Hematol Oncol Clin North Am. 1996;10:499-530.
- Manne V, Vaddi SP, Reddy VB, Dayapule S. Factors influencing patency of brescia-cimino arteriovenous fistulas in hemodialysis patients. Saudi J Kidney Dis Transpl. 2017;28(2):313-17.
- Farber A, Tan TW, Hu B, Dember LM, Beck GJ, Dixon BS, et al. Dialysis Access Consortium (DAC) study group; dialysis access consortium DAC study group. The effect of location and configuration on forearm and upper arm hemodialysis arteriovenousgrafts. J Vasc Surg. 2015;62(5):1258-64.
- Bender MH, Bruyninckx CM, Gerlag PG. The brachiocephalic elbow fistula: A useful alternative angioaccess for permanent hemodialysis. J Vasc Surg. 1994;20(5):808-13.
- Kaygin MA, Talay S, Dag O, Erkut B. An experience of arteriovenous fistulas created for hemodialysis in the largest health center in eastern Turkey. Ren Fail. 2012;34(3):291-6.
- Koo Seen Lin LC, Burnapp L. Contemporary vascular access surgery for chronic hemodialysis. J R Coll Surg Edinb. 1996;41:164-69.